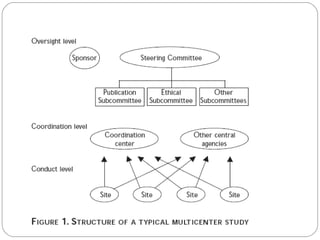
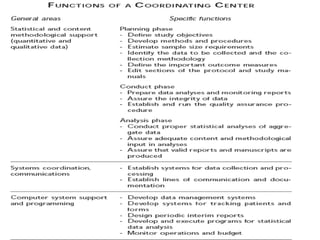
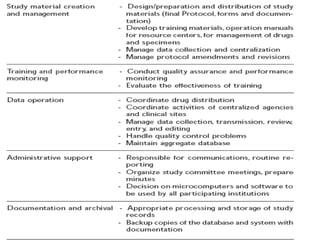
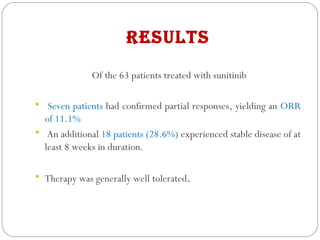
This document discusses multicentric clinical trials. It begins by defining clinical trials and introducing that a multicenter trial is conducted across multiple medical centers. Key points are that multicenter trials require standardization of procedures, uniformity, high data quality, and collaboration across sites. The document distinguishes between multi-site and multicentric studies, noting that in multicentric studies investigators at sites are co-investigators in planning and responsible for results, while in multi-site studies sites merely carry out tasks. Coordination in multicenter studies involves centralized activities like protocol development and data management to standardize procedures. Advantages include larger sample sizes and evaluating efficacy across populations. The document concludes by summarizing a phase II multicenter trial of sunit