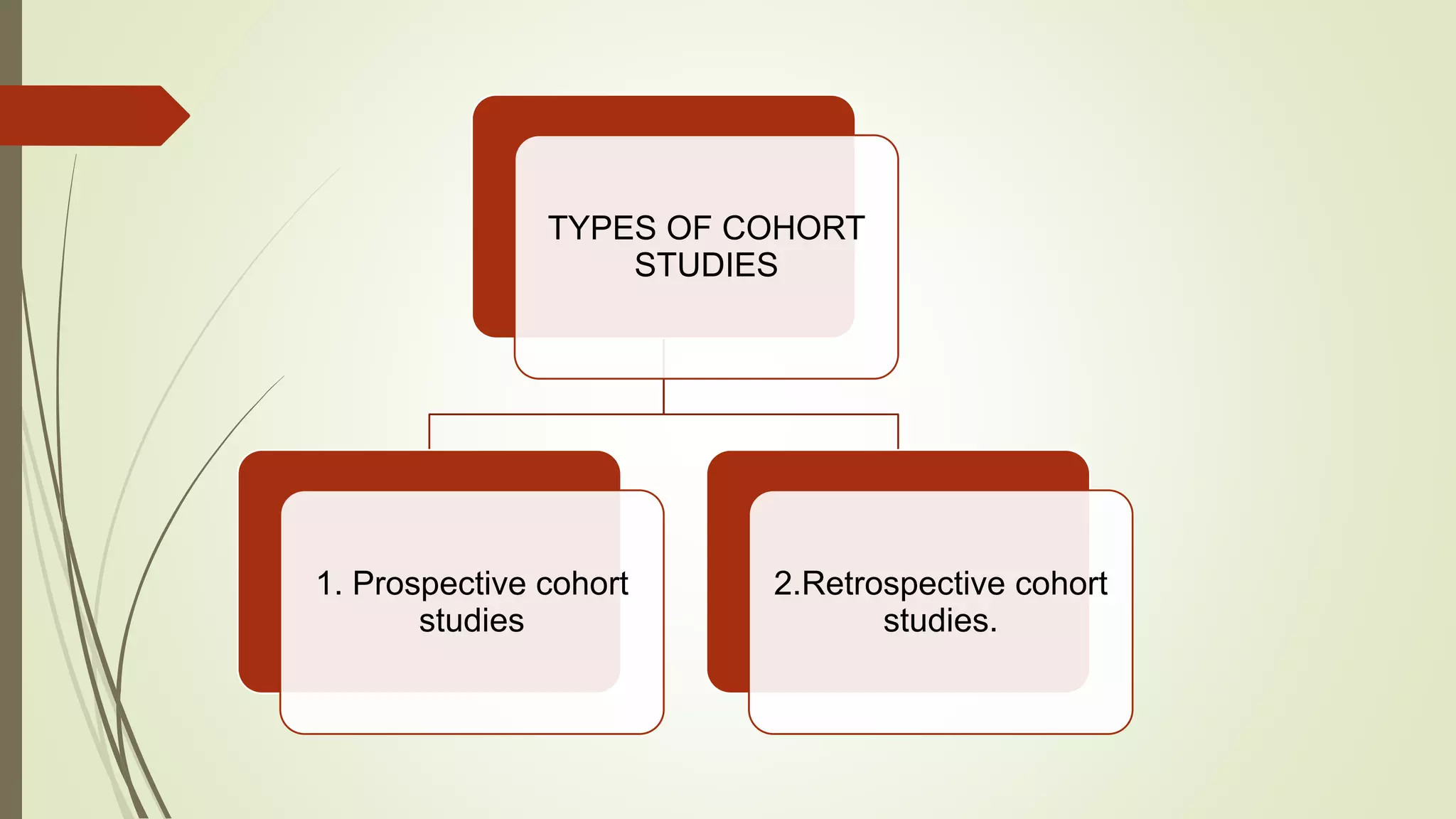
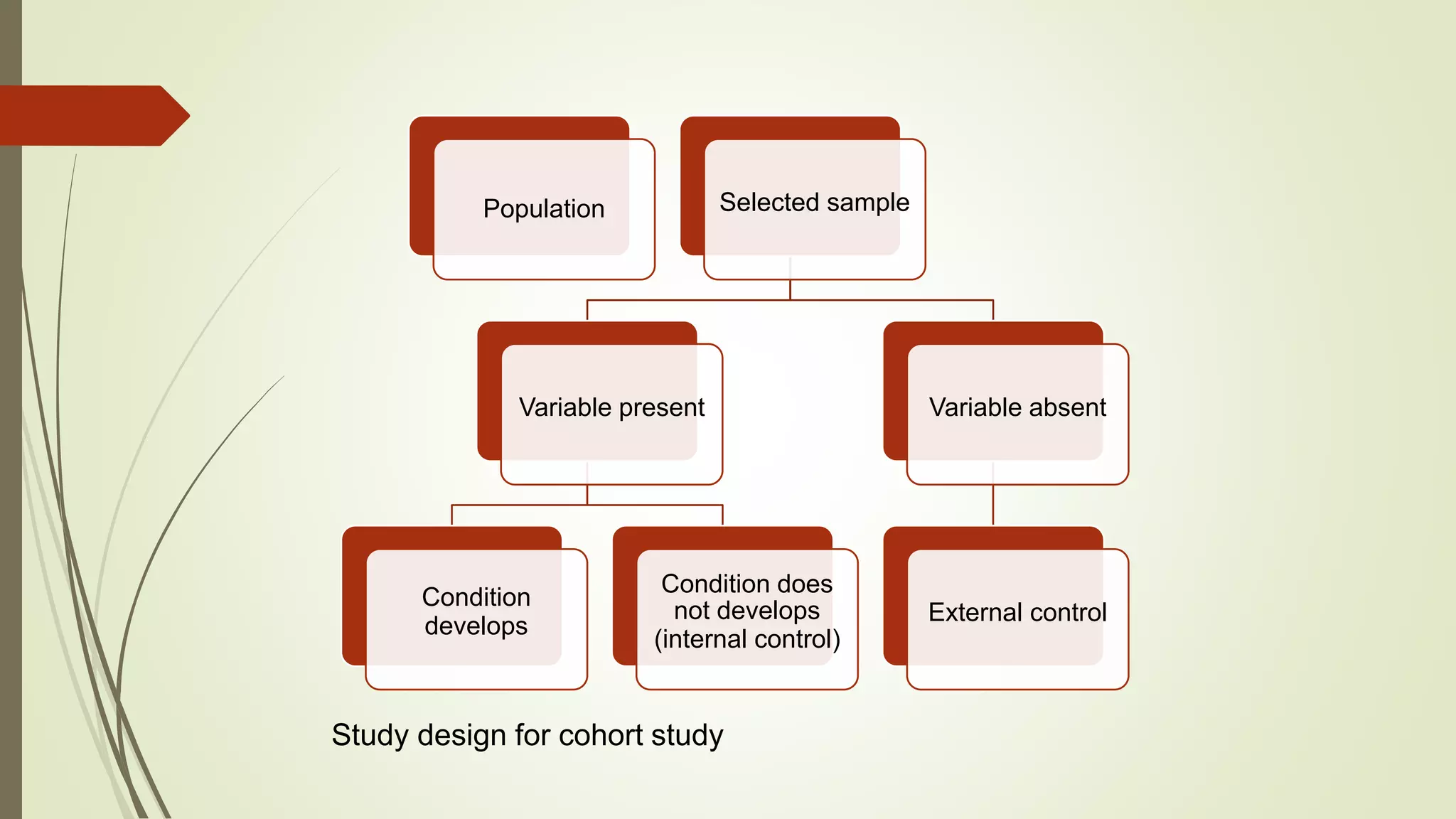
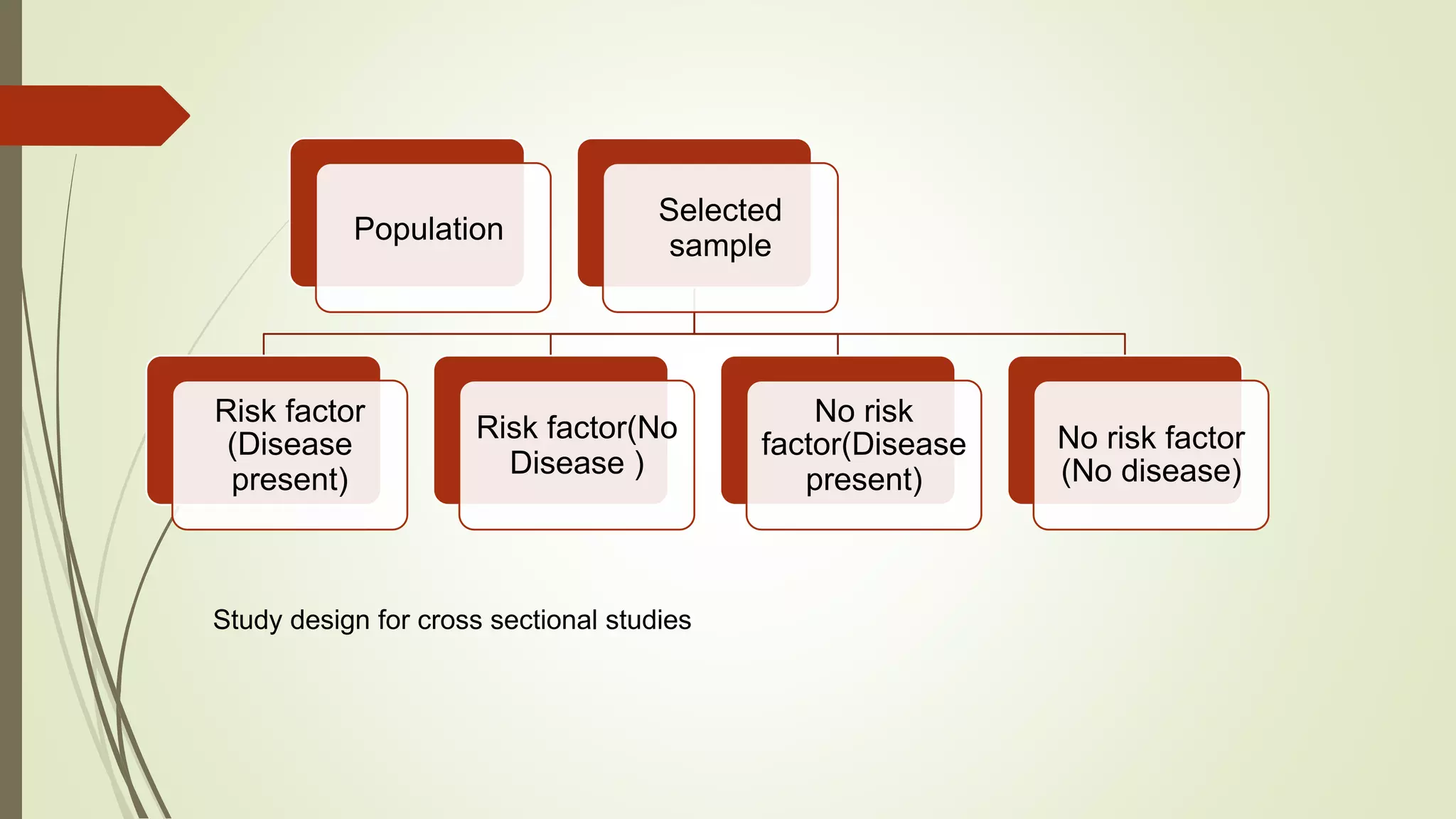
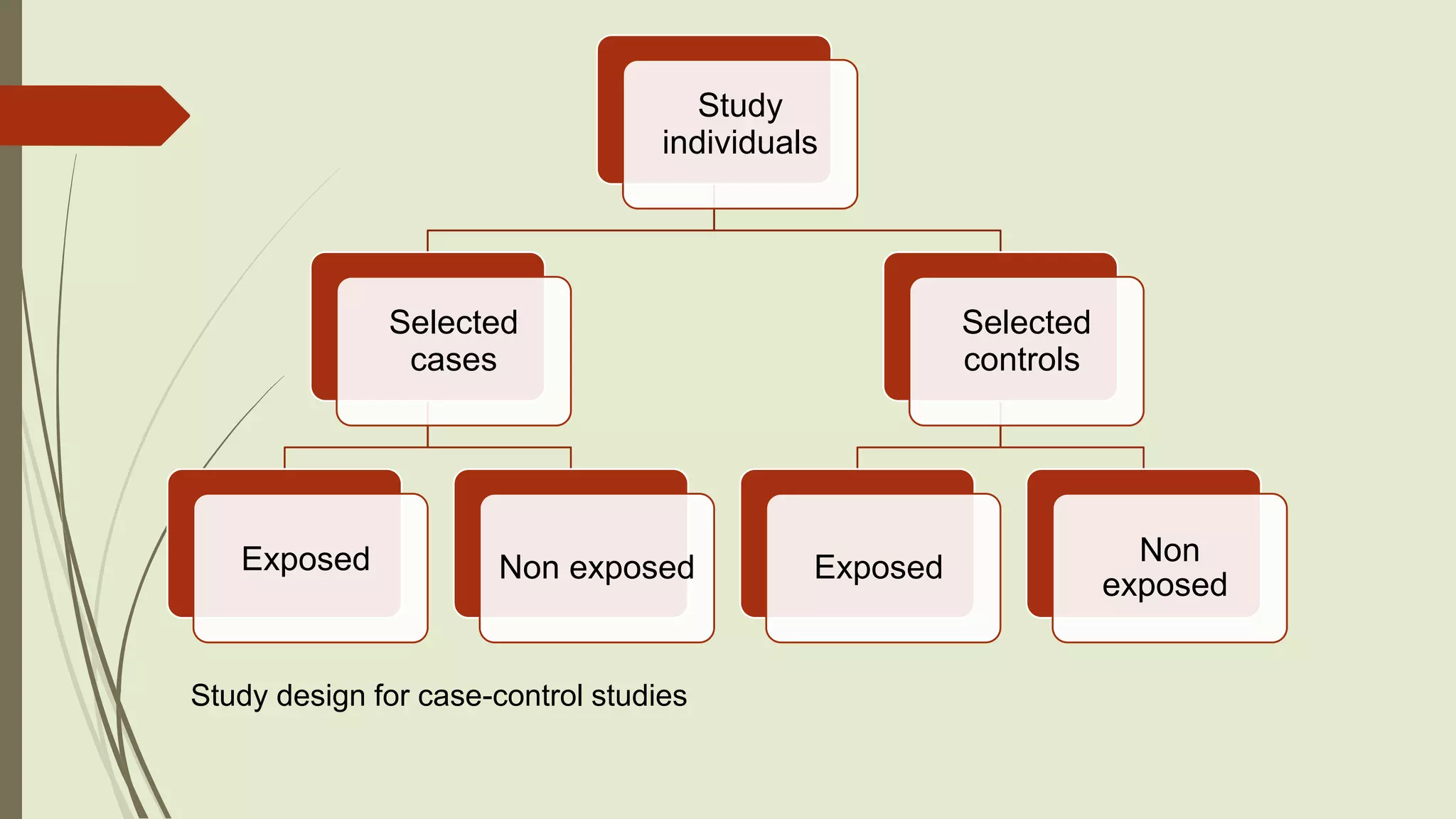
This document provides an overview of observational study designs, including definition, types, and examples. It discusses cohort studies, case-control studies, and cross-sectional studies. Cohort studies measure events over time to determine causes and prognosis. Case-control studies identify risk factors by comparing exposed and unexposed groups. Cross-sectional studies analyze a population at a single time point to determine prevalence. Observational studies are useful when randomized controlled trials are unethical or for studying rare conditions and adverse events.