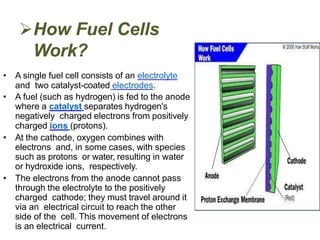
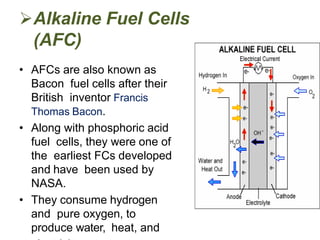
This document provides information on fuel cells and specifically discusses alkaline fuel cells (AFCs). It describes that AFCs use an aqueous alkaline electrolyte, such as potassium hydroxide, and consume hydrogen and oxygen to produce electricity, water, and heat. AFCs have a similar construction to batteries with two electrodes separated by an electrolyte-soaked matrix. They are very sensitive to carbon dioxide and operate at temperatures of 150-200 degrees Celsius. Some advantages of AFCs are their low manufacturing costs due to inexpensive catalyst materials and efficiencies up to 70%.