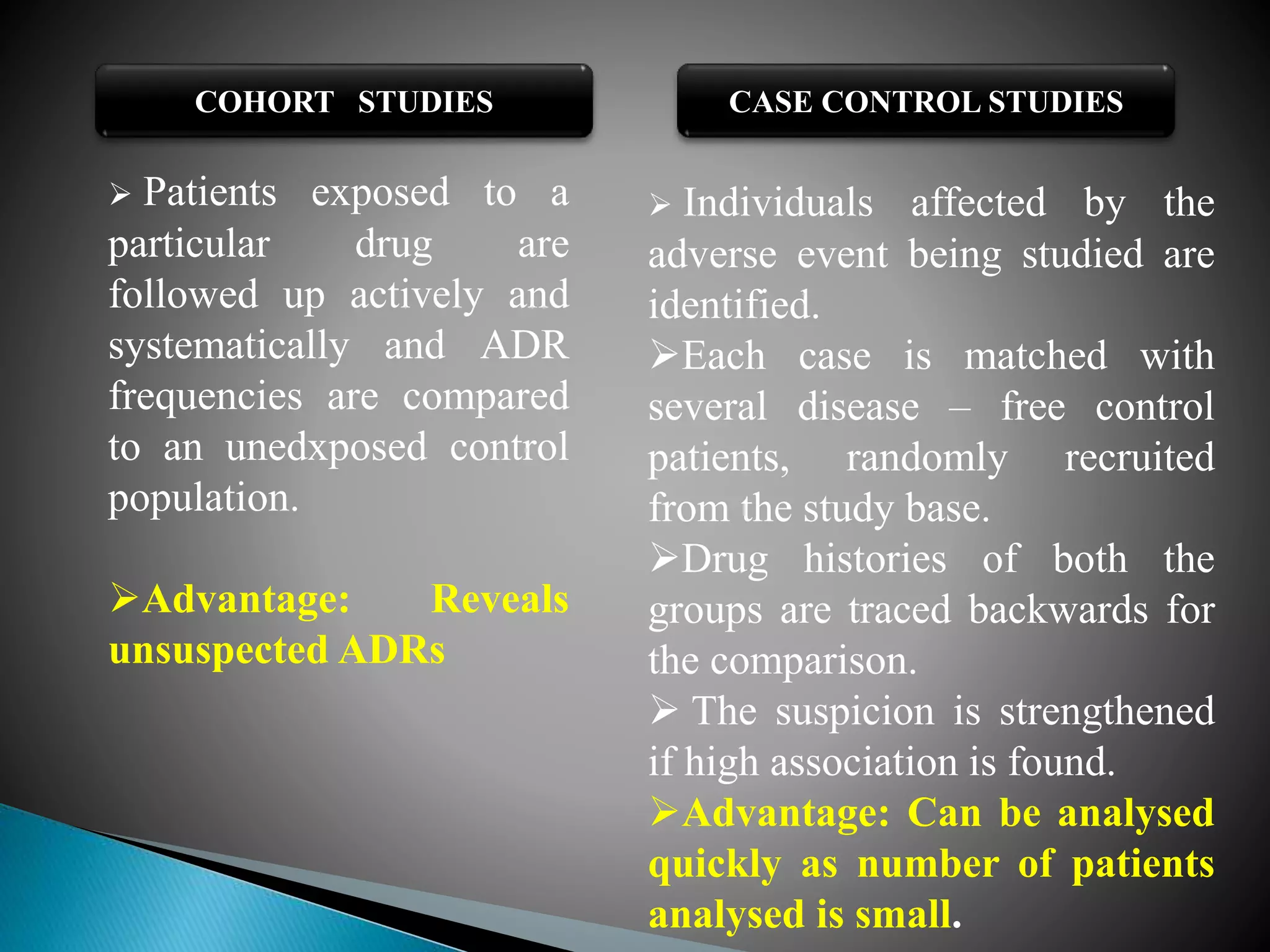
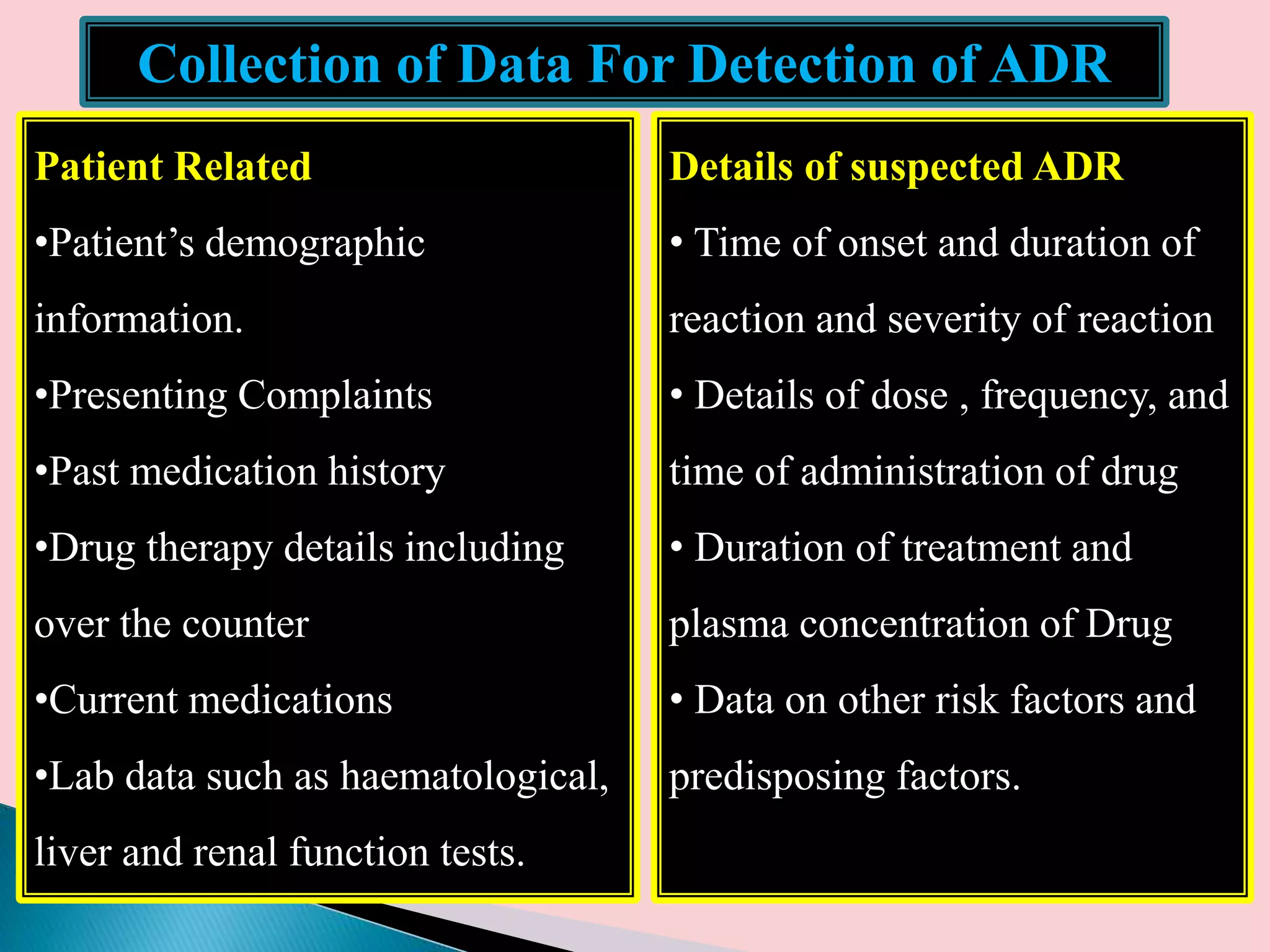
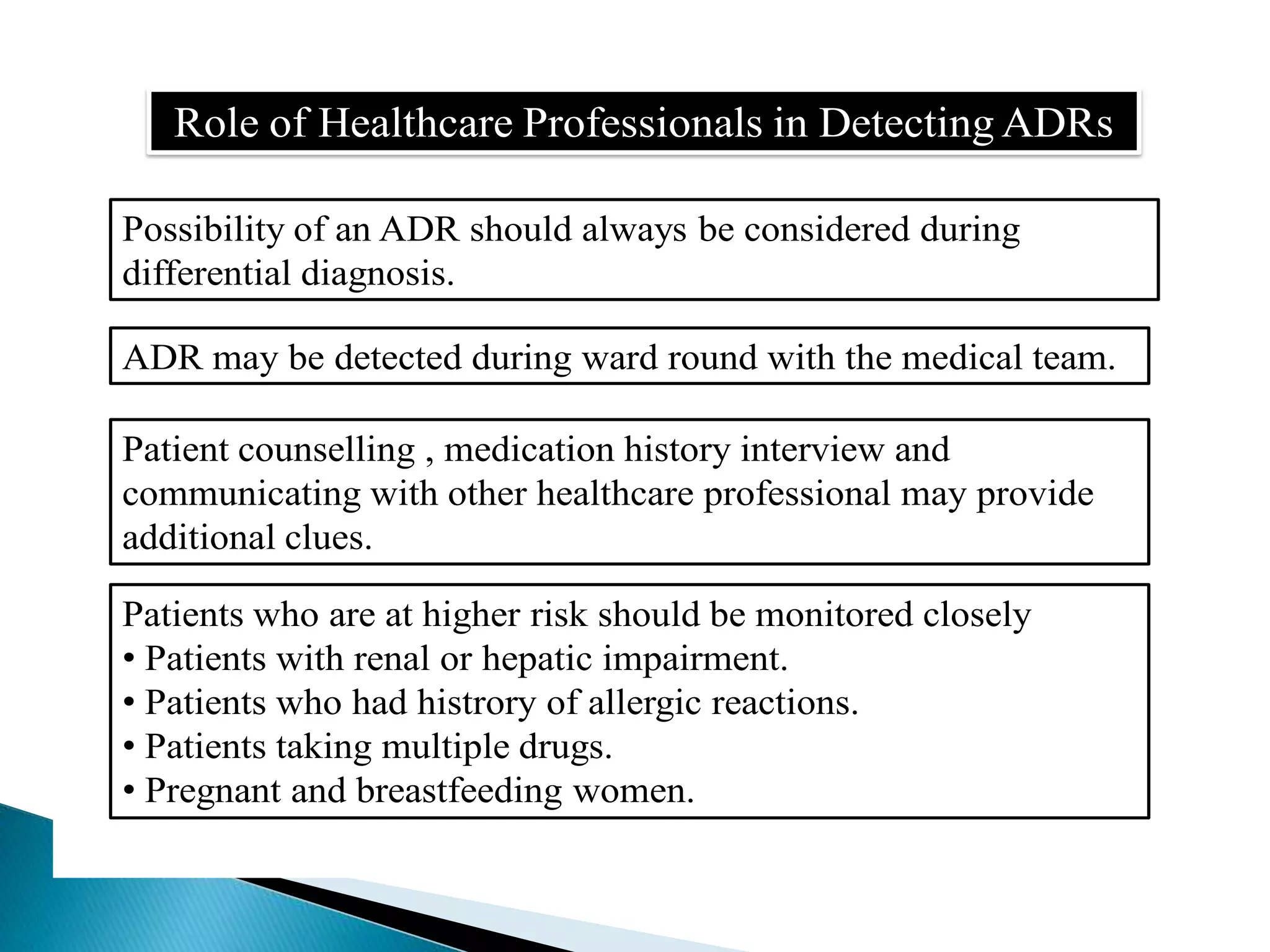
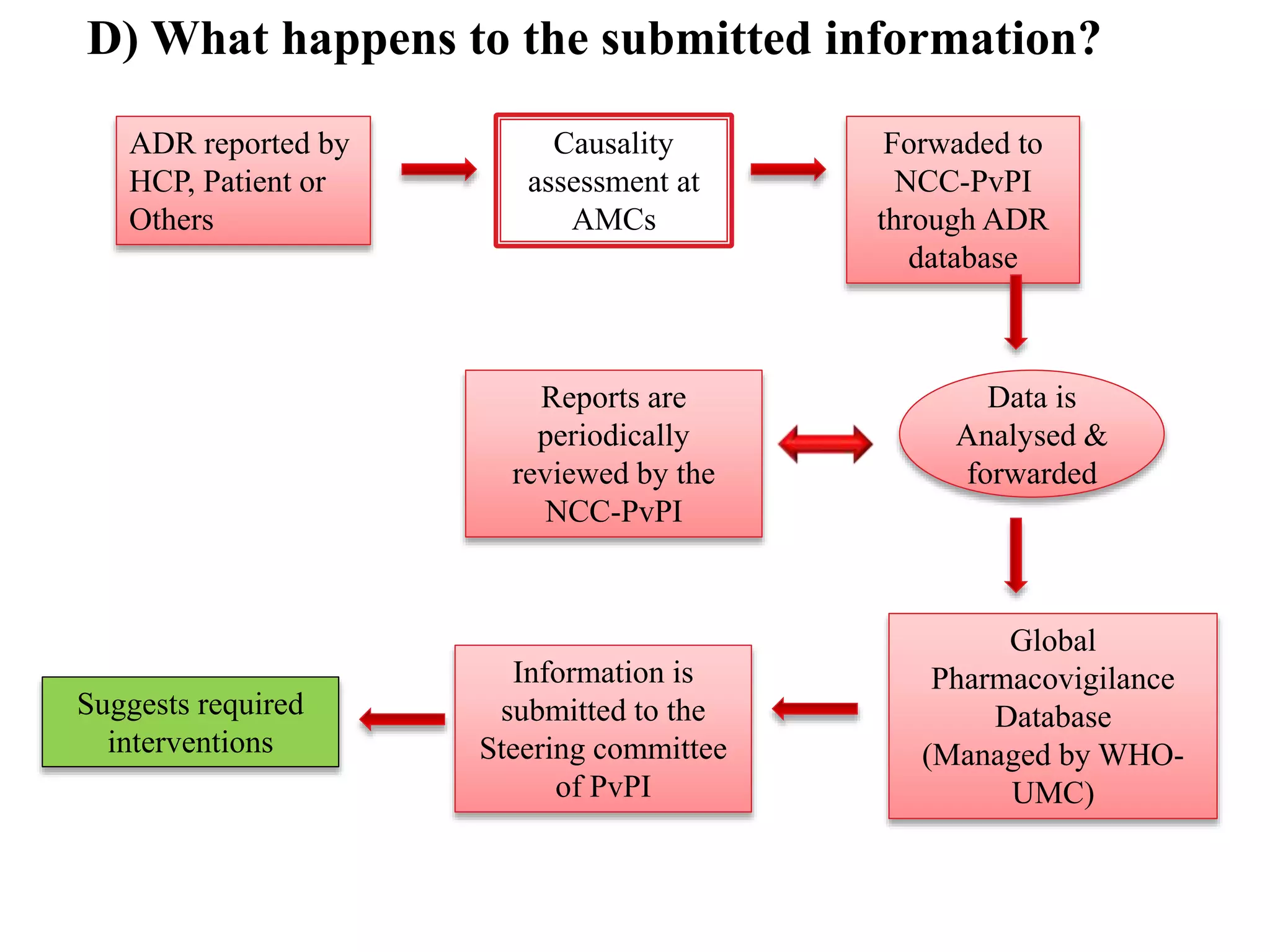
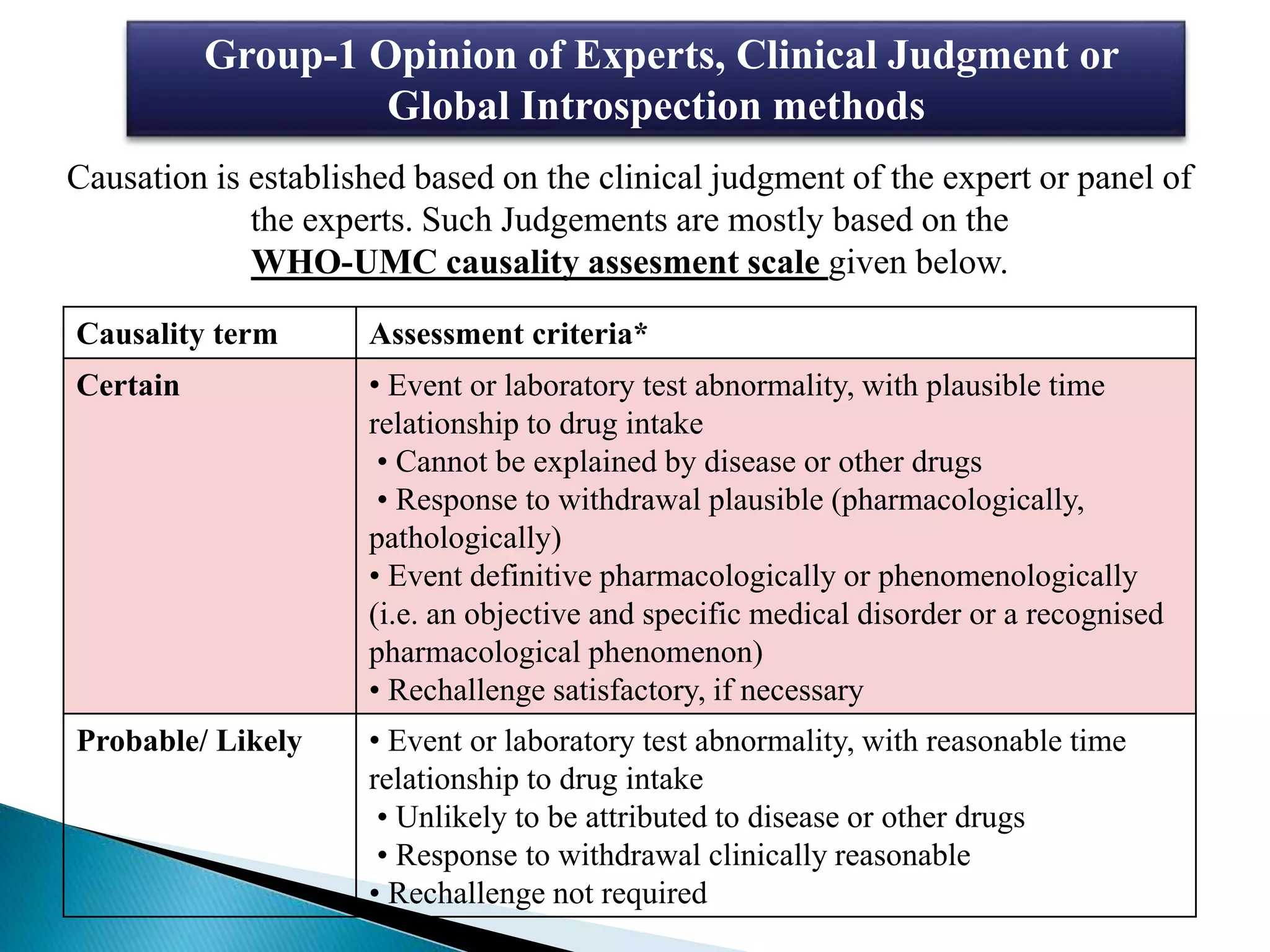
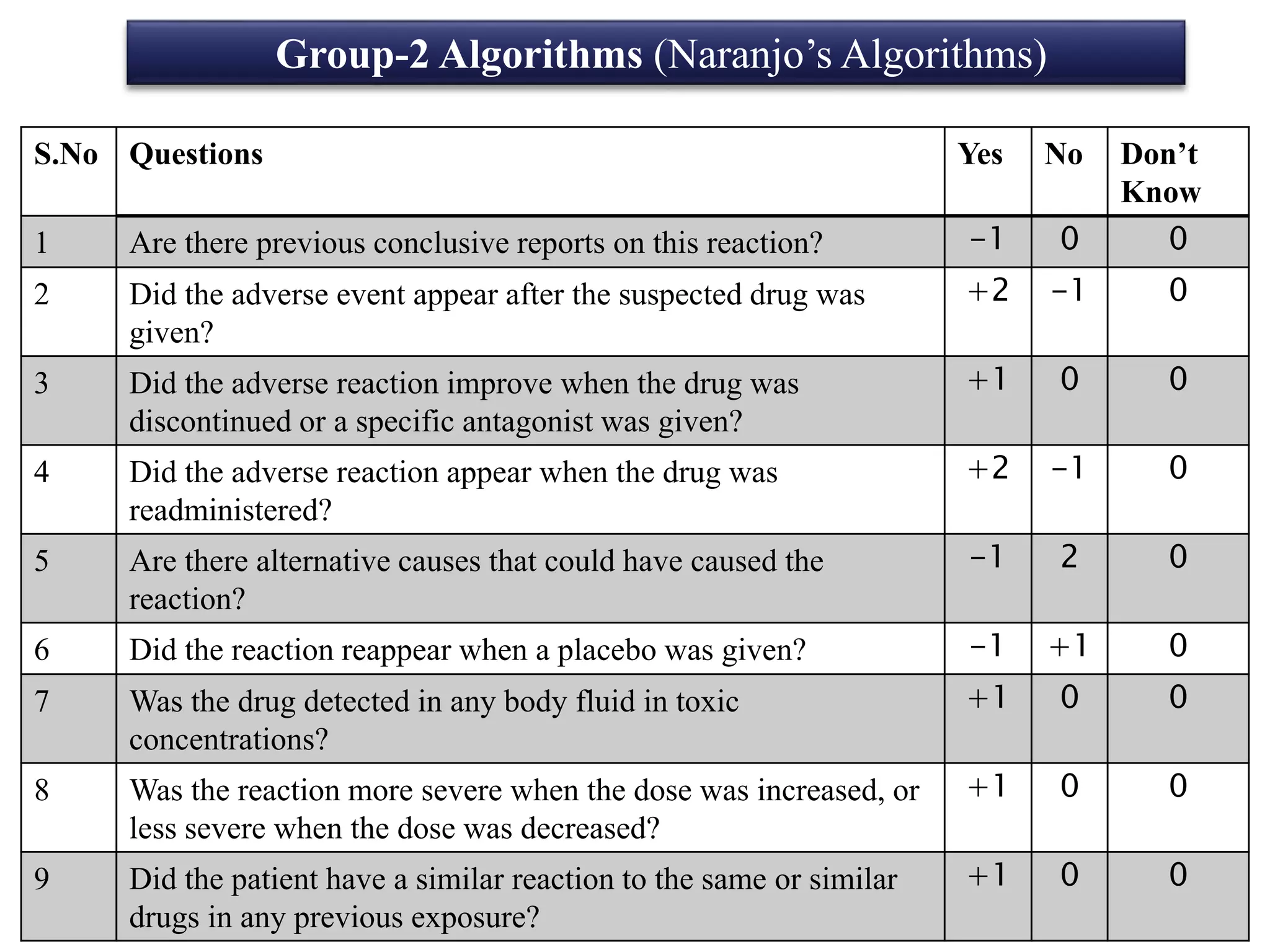
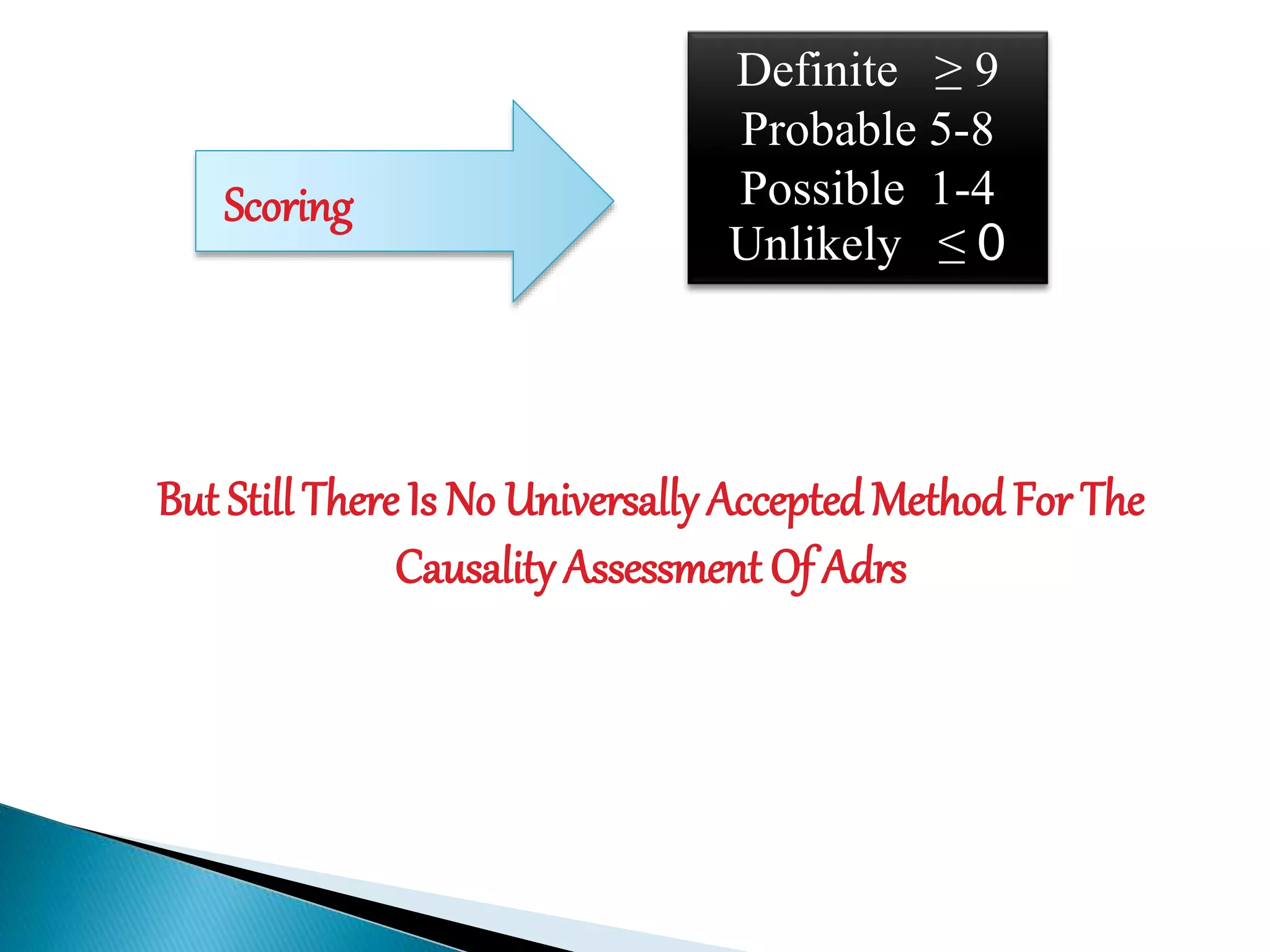
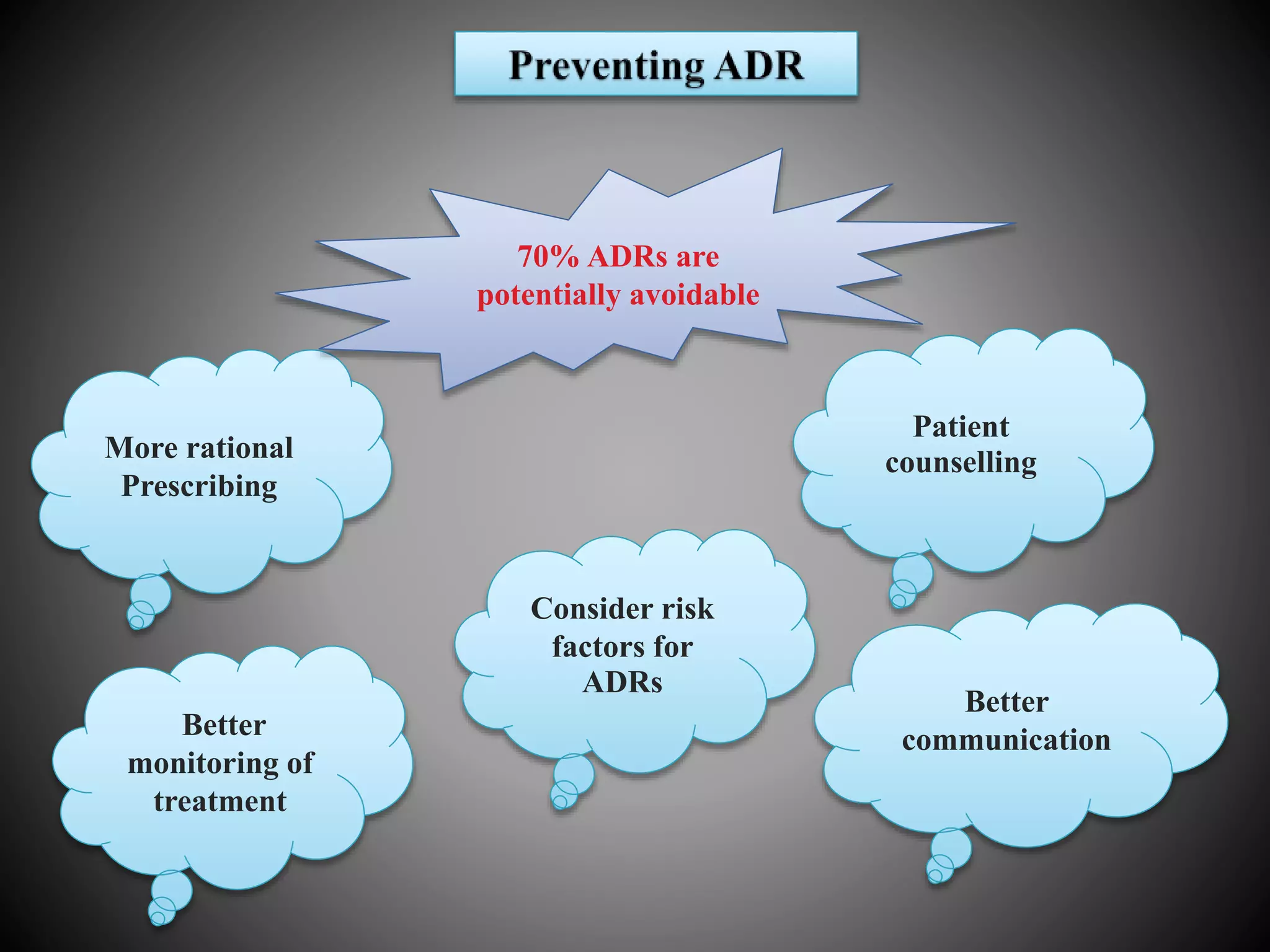
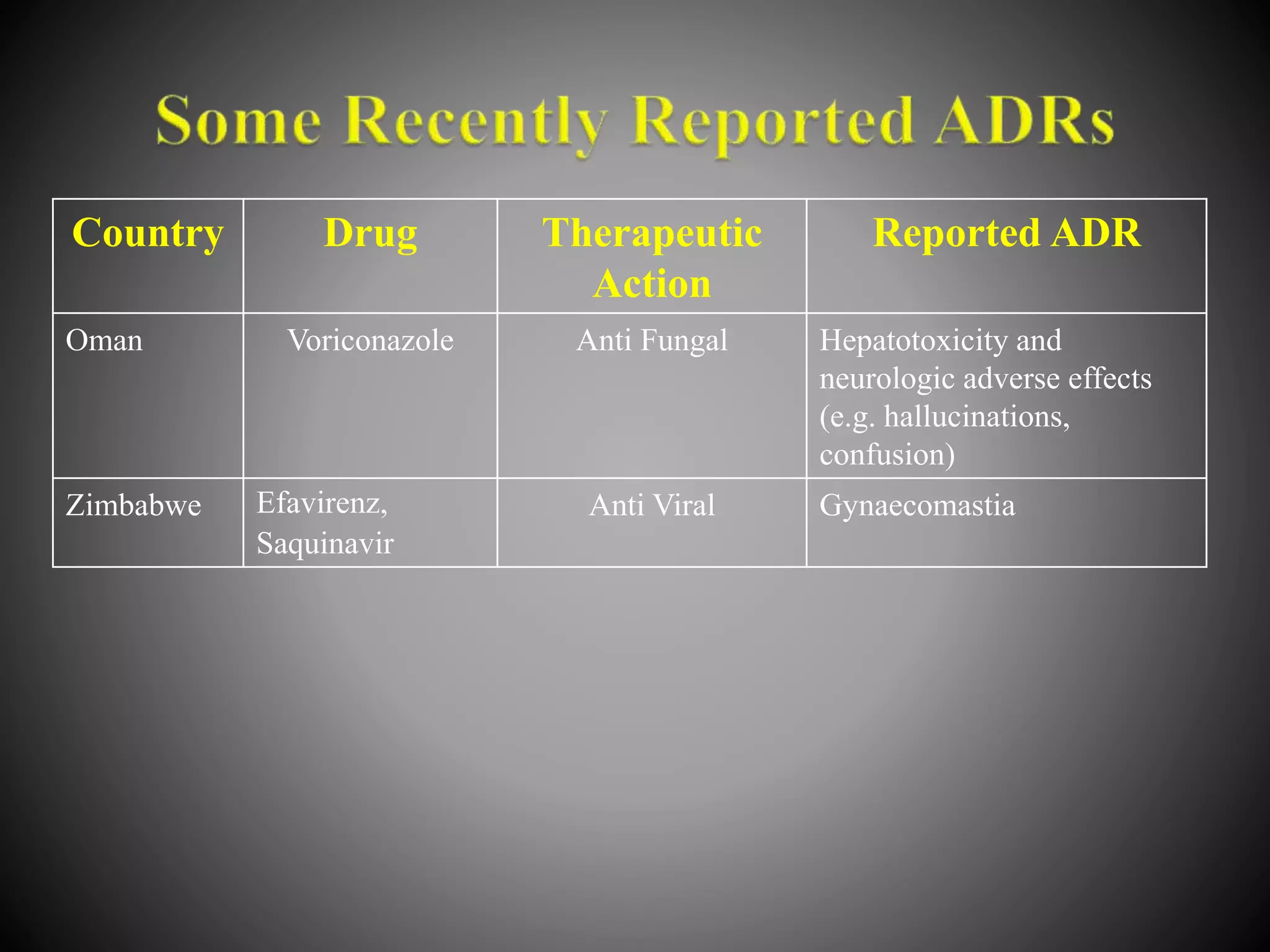
The document discusses adverse drug reactions (ADRs), defining them as noxious and unintended responses to drugs used in standard dosages, according to WHO. It covers the classification, detection, management, reporting of ADRs, and the roles of healthcare professionals in identifying them. Special emphasis is placed on causality assessment and recommendations for reporting suspected ADRs to pharmacovigilance centers.