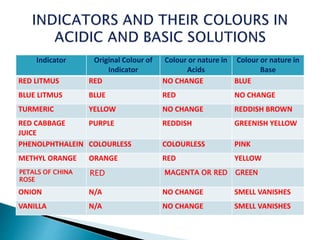
The document discusses various types of indicators used to determine the acidic or basic nature of solutions through color changes. It covers universal, natural, olfactory, and synthetic indicators, providing examples such as litmus, turmeric, and phenolphthalein. Each indicator is described with its original color and the color it turns in acidic or basic solutions.