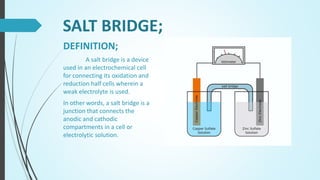
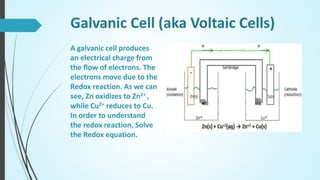
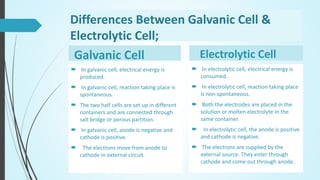
Saman Tanoli will present on electrochemical cells. The presentation will define electrochemical cells, describe their components and types, including voltaic/galvanic cells and electrolytic cells. It will explain the differences between galvanic and electrolytic cells and provide examples of their applications. Voltaic cells generate electricity from spontaneous redox reactions, while electrolytic cells use electricity to drive non-spontaneous reactions. Common examples are batteries and electroplating/electrorefining of metals.