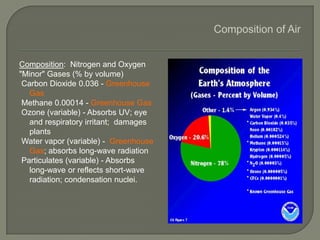
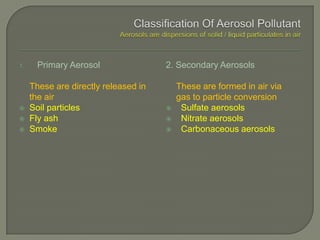
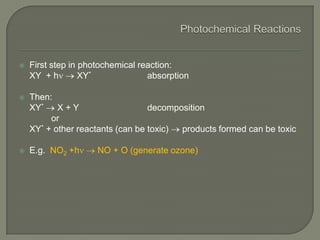
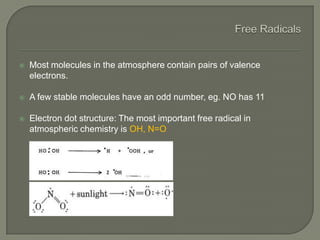
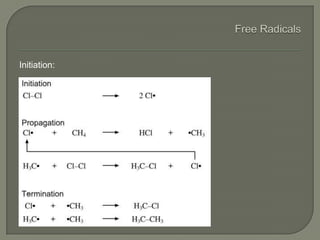
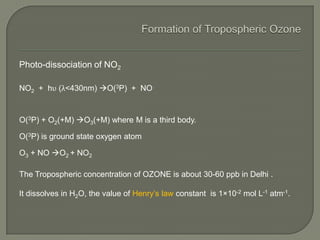
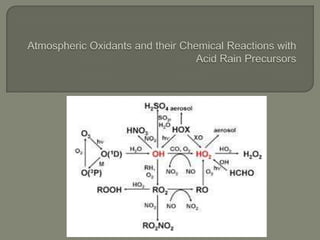
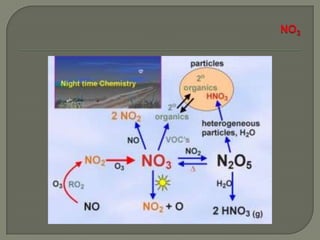
The document discusses air pollution and its causes. It notes that while oxygen and nitrogen make up most of the atmosphere, trace gases like carbon dioxide, methane, and ozone also play important roles. It describes various natural and human-caused sources of air pollution including industry, transportation, and the burning of fossil fuels. The document outlines primary and secondary pollutants as well as different types of particulate matter. It also discusses the chemistry of pollutants in the atmosphere and their interactions with sunlight and water.





























![ In dust – free and unpolluted atmosphere pH of rain water and other
aqueous systems is determined by following reactions:
CO2(g) + H2O ↔ CO2
.H2O; KH = 4.5x10-2 mol L-1 atm-1
CO2
.H2O ↔H+ + HCO3
-; K1 = 3.8х10-7 mol L-1 (carbonic acid)
These Eqs. show, [H+] = ( K1KHpCO2)½ , With = pCO2 =380 ppm = 3.8 х 10-4
atm, one gets, pH = ~ 5.6
So Reference or Background pH for Rain Water = 5.6, If the pH of rain
water is less than 5.6, it is called acid rain](https://image.slidesharecdn.com/atnmosphericoxidants-140328005939-phpapp02/85/Atmospheric-Oxidants-38-320.jpg)





