1. Acids are hydrogen ion donors and bases are hydrogen ion acceptors. The acid-base balance can be affected by both strong acids and bases that dissociate completely in solution as well as weak acids and bases that only partially dissociate.
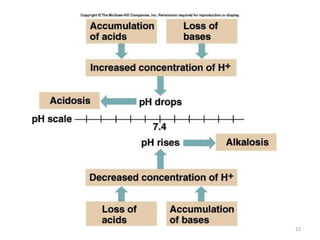
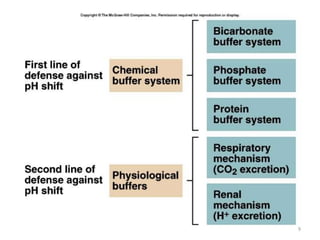
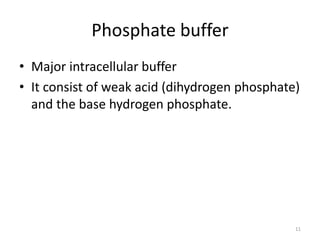
2. The pH measures the concentration of hydrogen ions and small changes in pH can significantly impact enzyme function and electrolyte levels. Buffers like bicarbonate and phosphate work to maintain acid-base balance by absorbing or releasing hydrogen ions as conditions change.
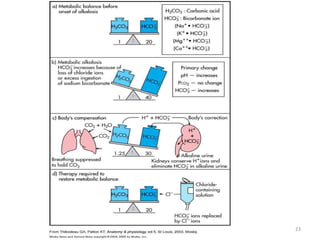
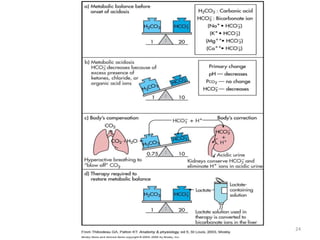
3. Respiratory and renal systems help regulate acid-base balance. Respiratory acidosis results from carbon dioxide excess while respiratory alkalosis is due to low carbon dioxide levels. Metabolic acidosis
![ACID – BASE BALANCE
JOHNY WILBERT, M.Sc[N]
LECTURER,
APOLLO INSTITUTE OF HOSPITAL
MANAGEMENT AND ALLIED SCIENCE](https://image.slidesharecdn.com/acidbasebalance-180617102059/75/Acid-base-balance-1-2048.jpg)

![pH
• pH is and indirect measure of [H+]](https://image.slidesharecdn.com/acidbasebalance-180617102059/85/Acid-base-balance-5-320.jpg)
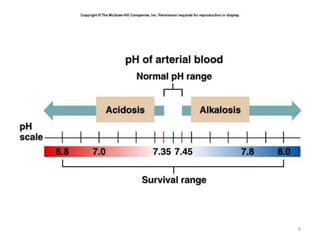



![Disorders of A-B balance
• Acidosis: abnormal condition lowering arterial pH
– before secondary changes in response to the primary aetiological
factor
• Alkalosis: abnormal condition raising arterial pH
– before secondary changes in response to the primary aetiological
factor
• Simple A-B disorders: there is a single primary aetiological
acid-base disorder
• Mixed A-B disorders: more primary aetiological disorders are
present simultaneously
Acidaemia: arterial pH<7.36 (i.e. [H+]>44 nM)
Alkalaemia: arterial pH>7.44 (i.e. [H+]<36 nM)](https://image.slidesharecdn.com/acidbasebalance-180617102059/85/Acid-base-balance-15-320.jpg)