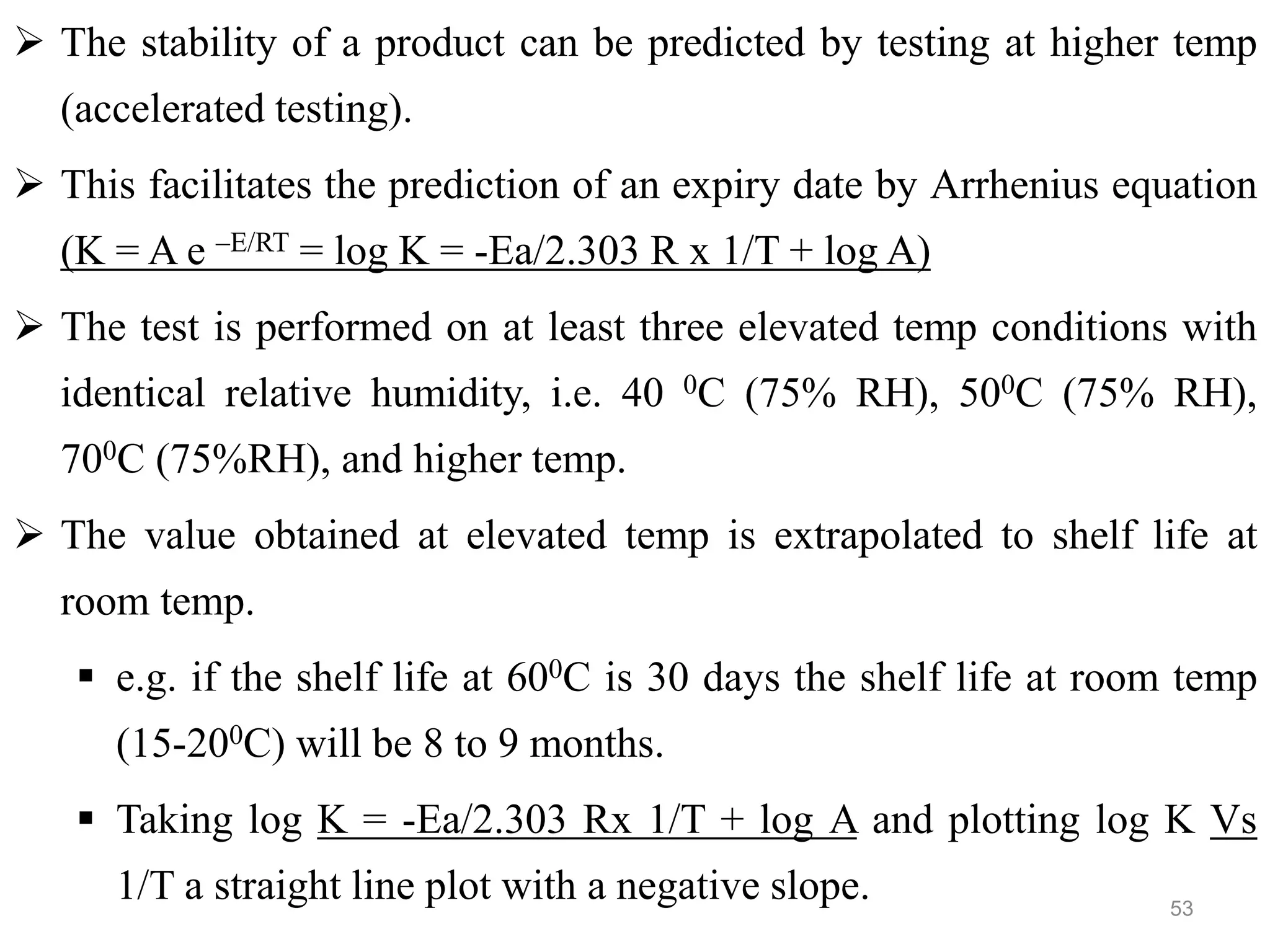
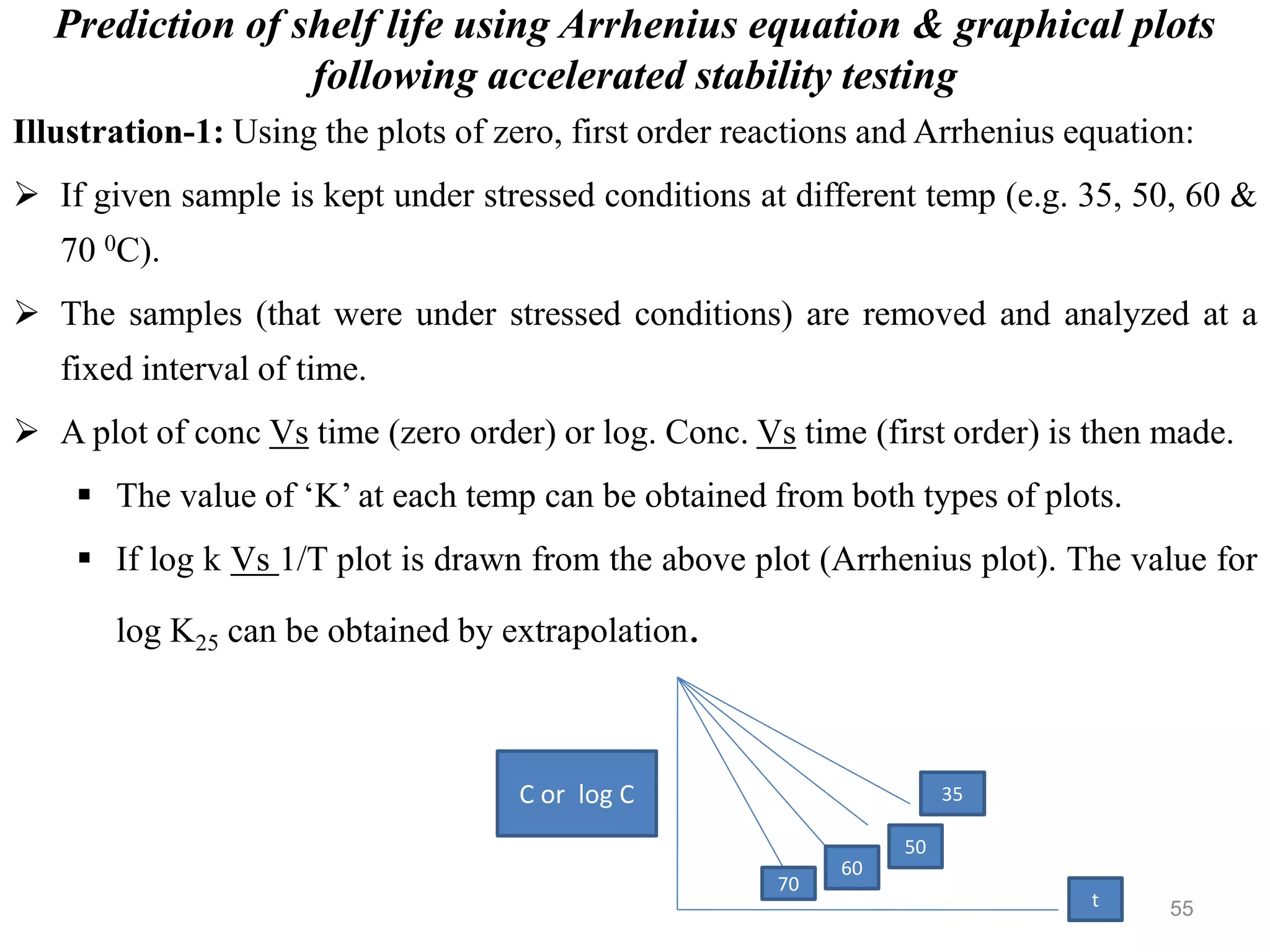
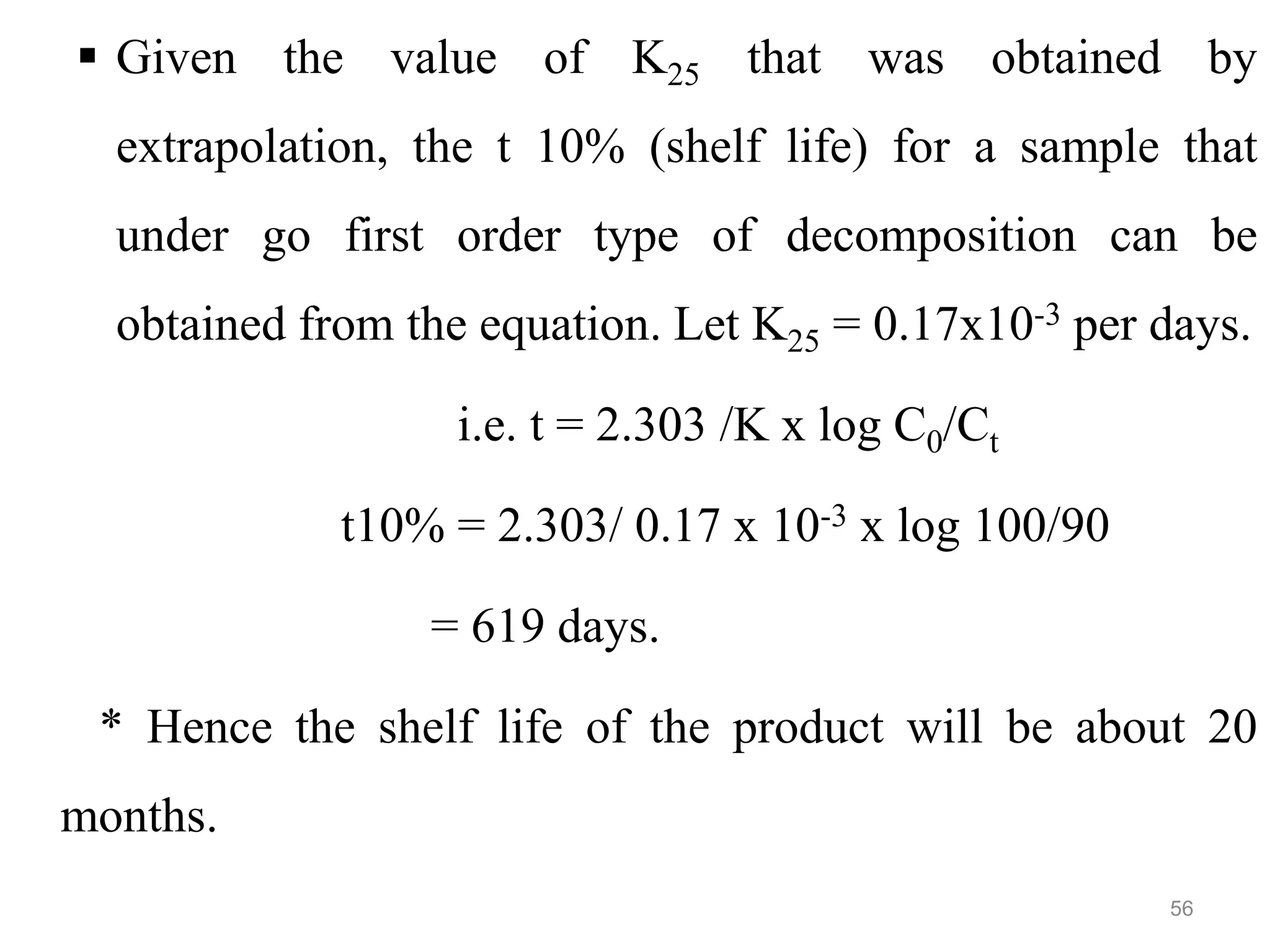
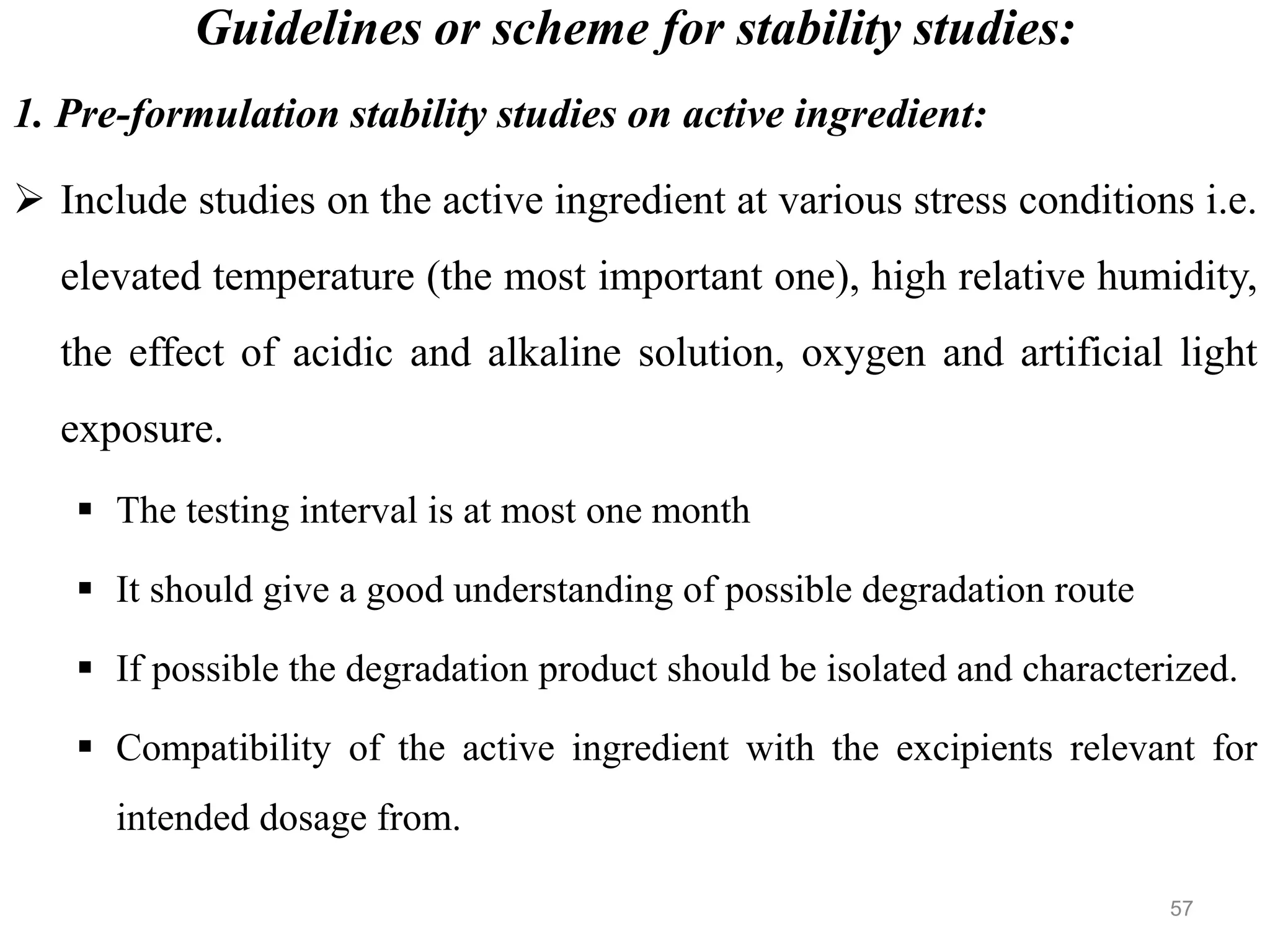
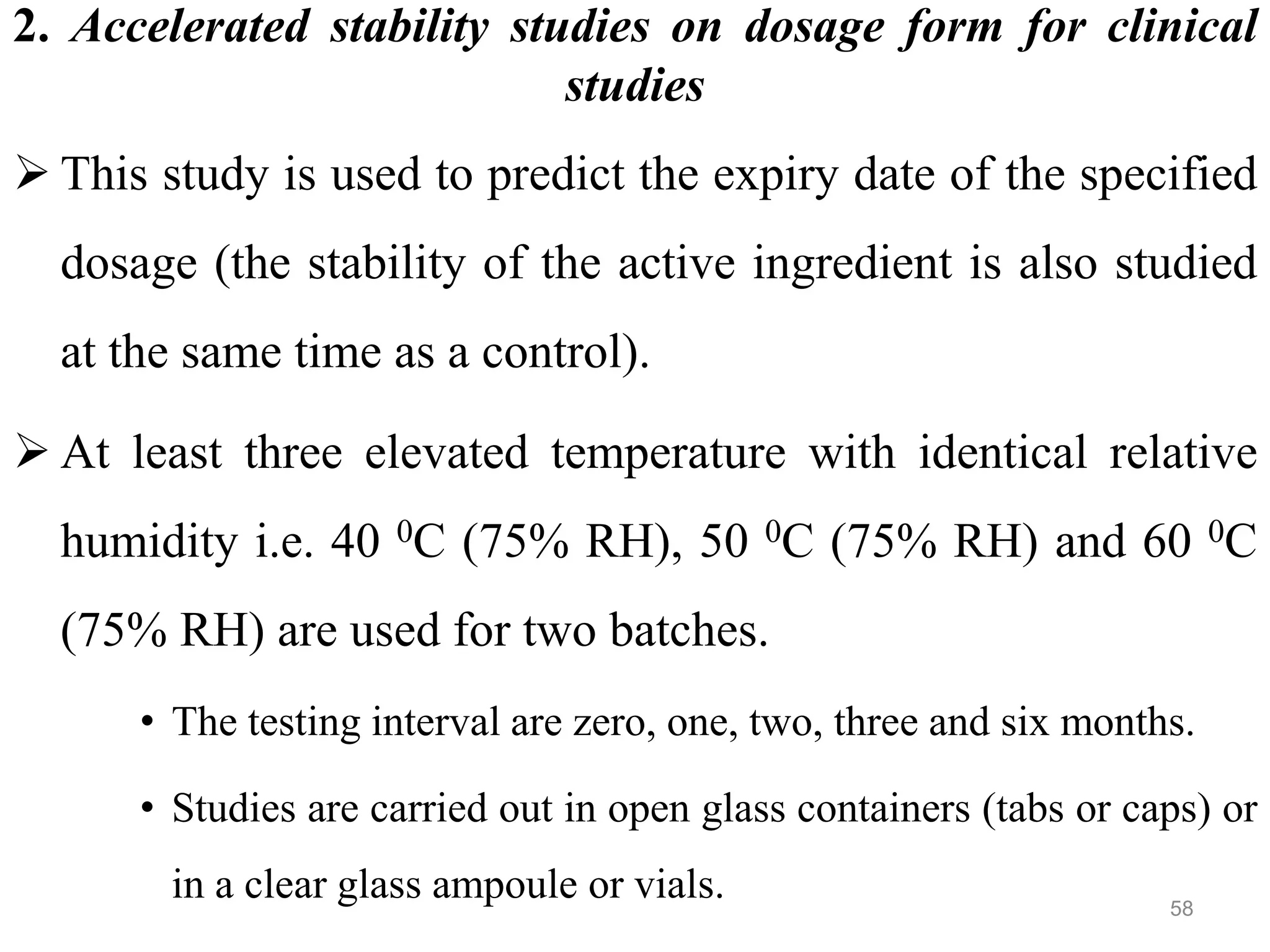
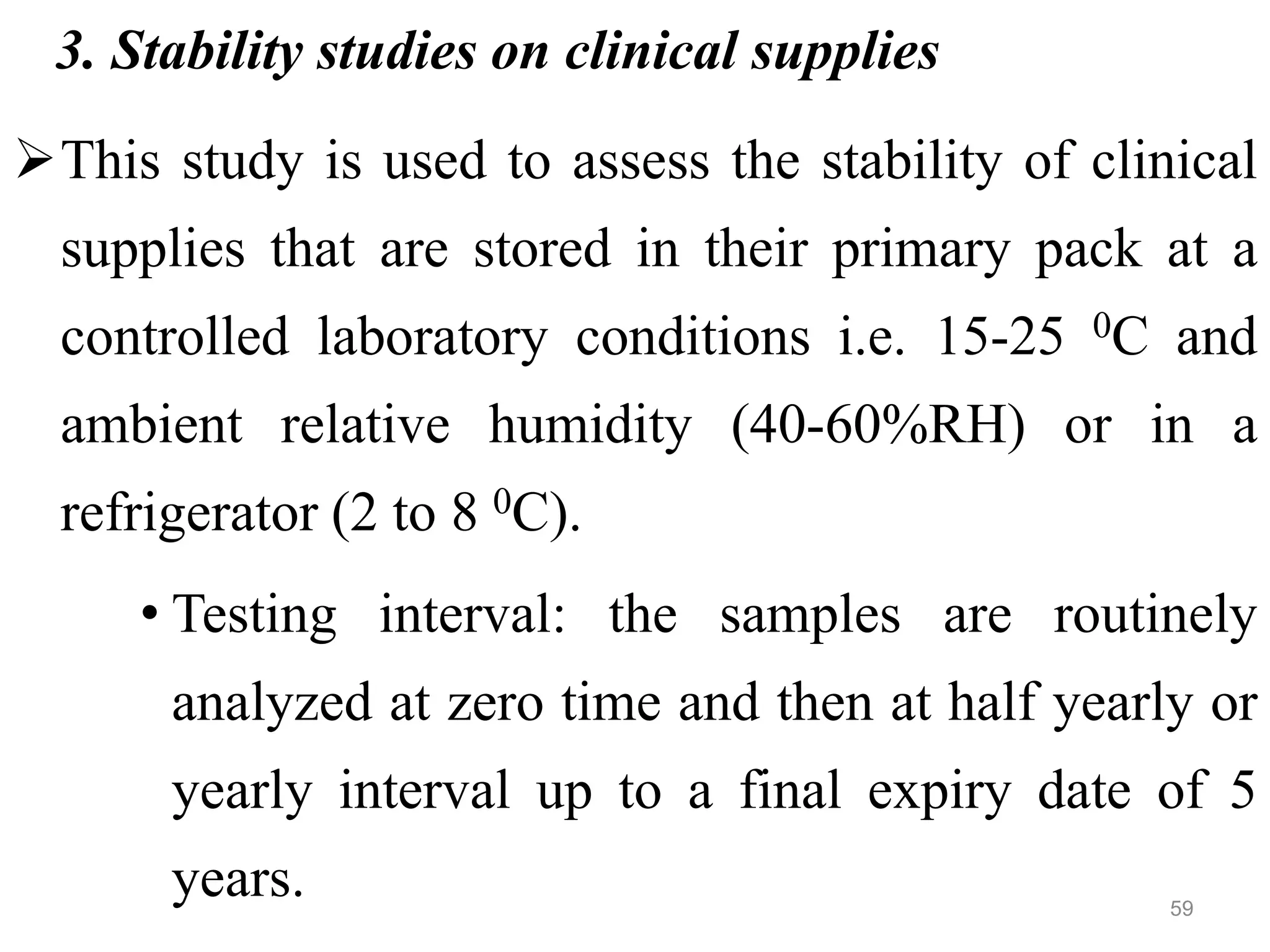
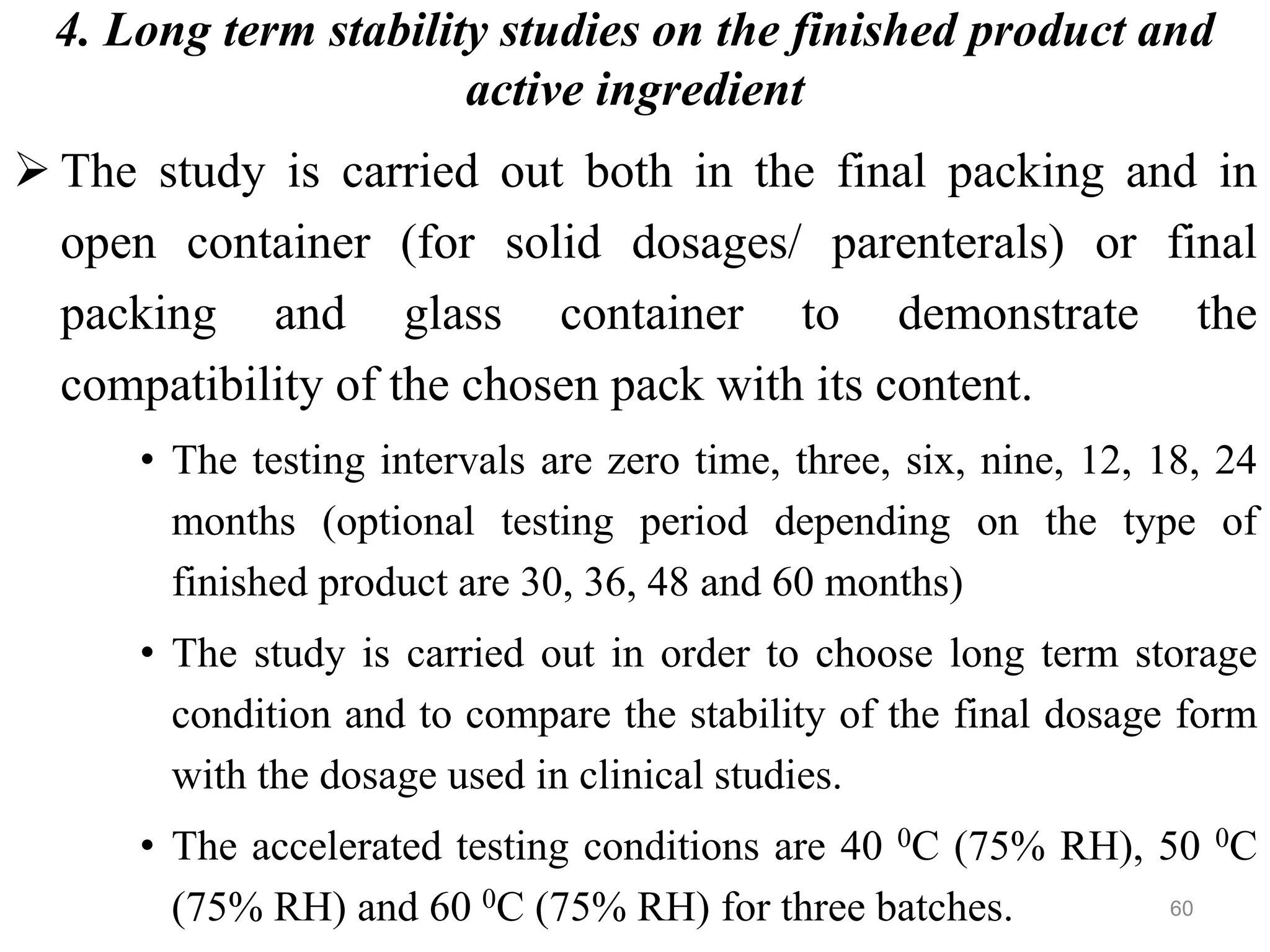
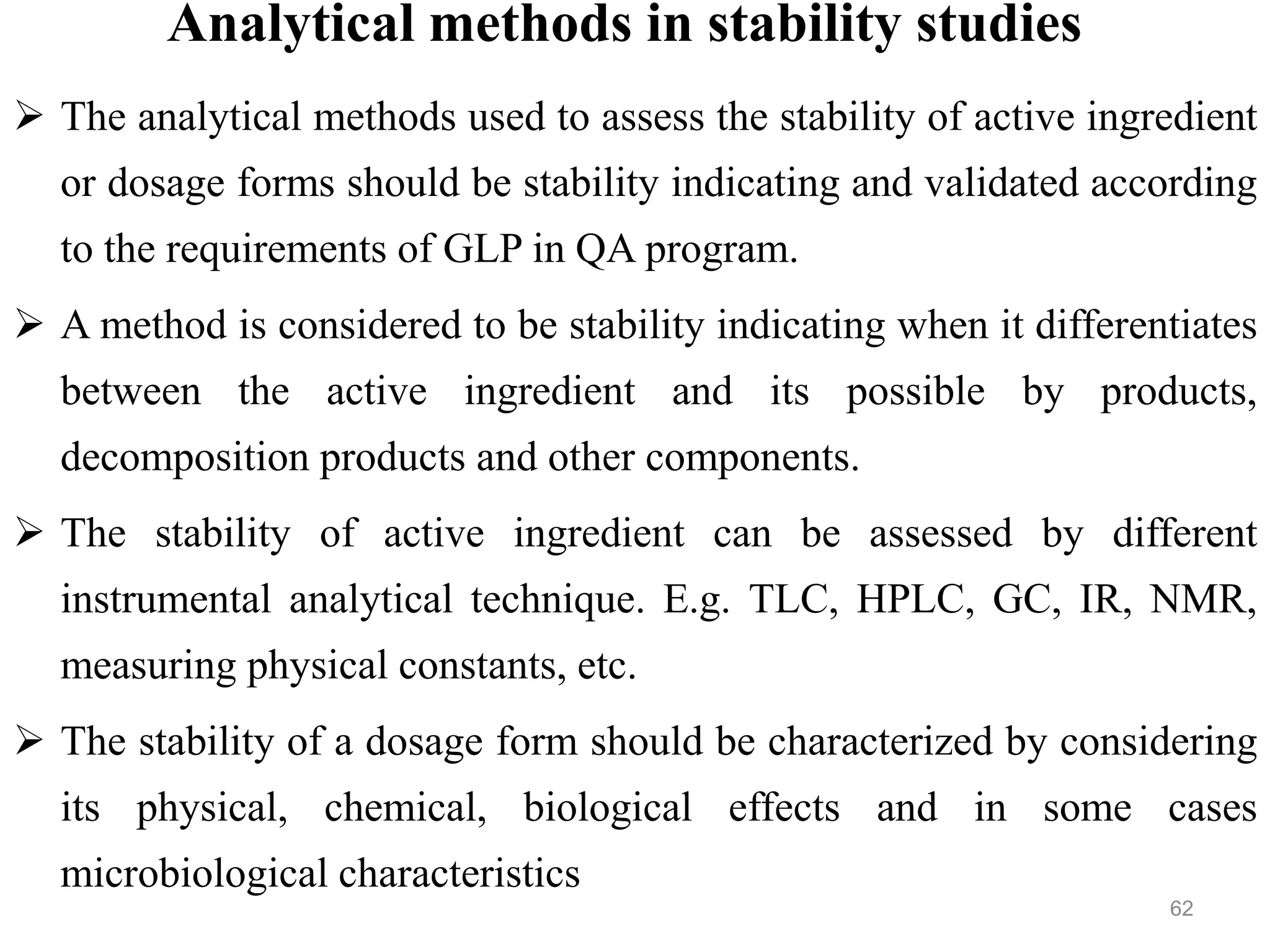
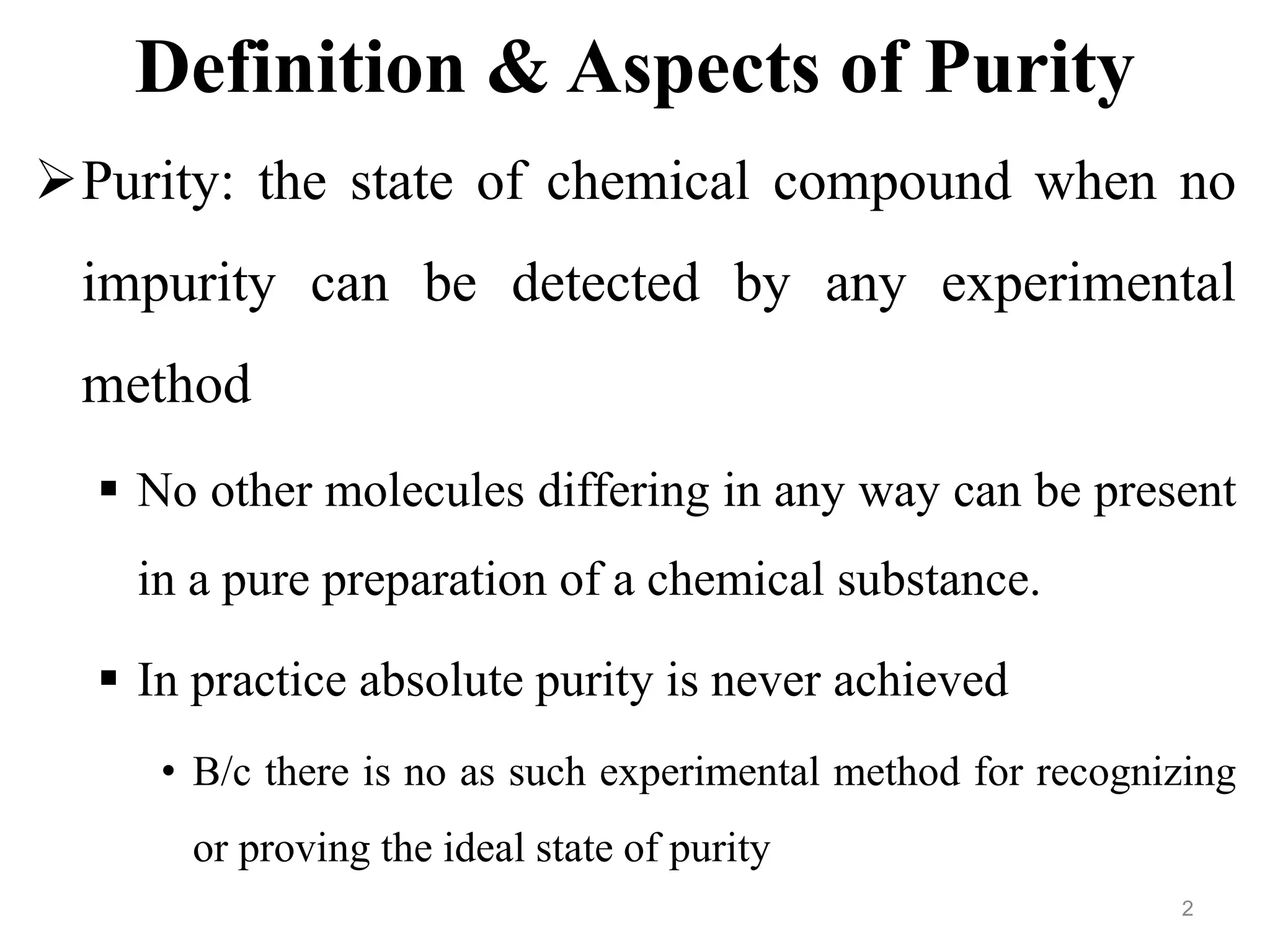
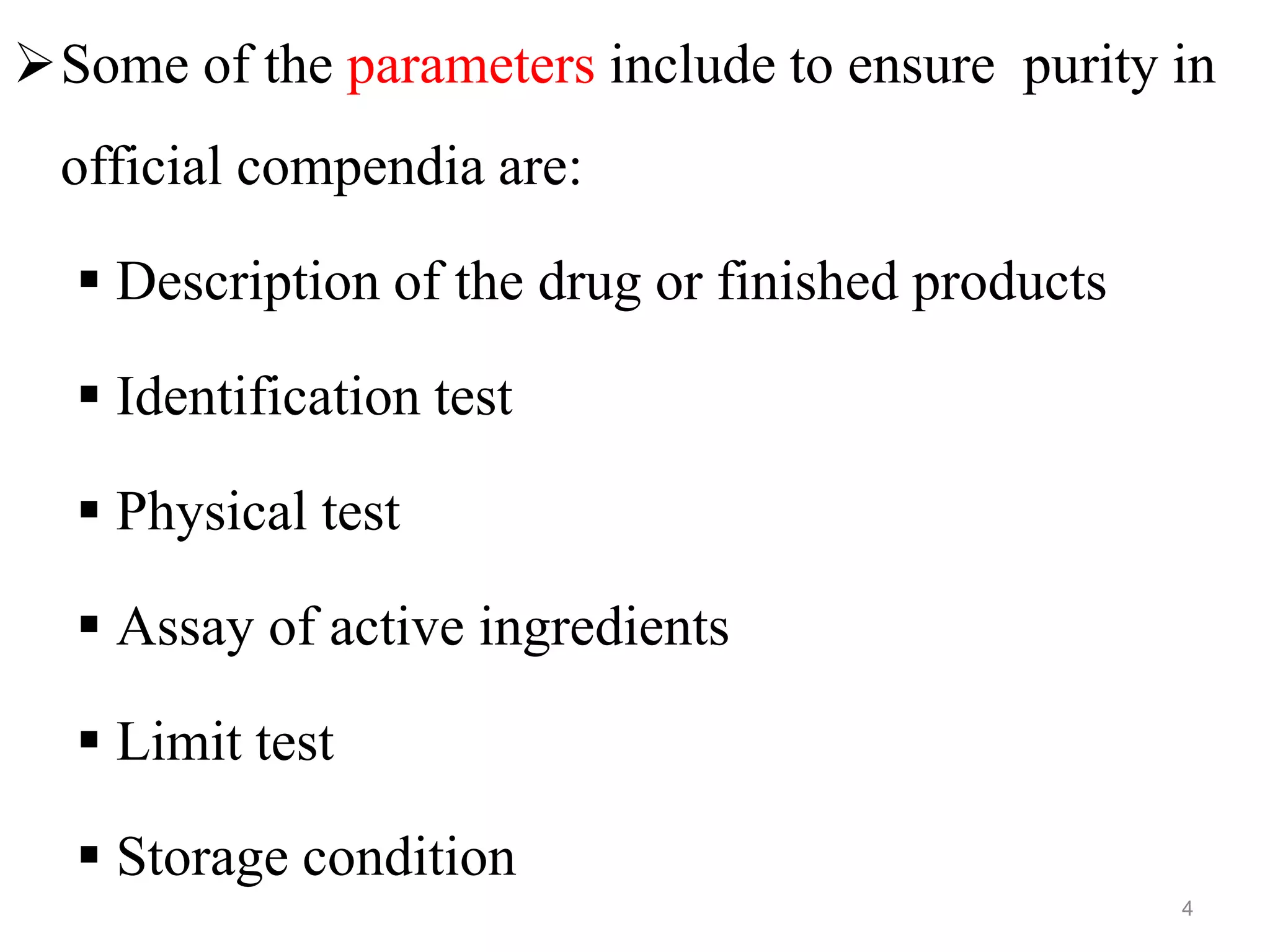
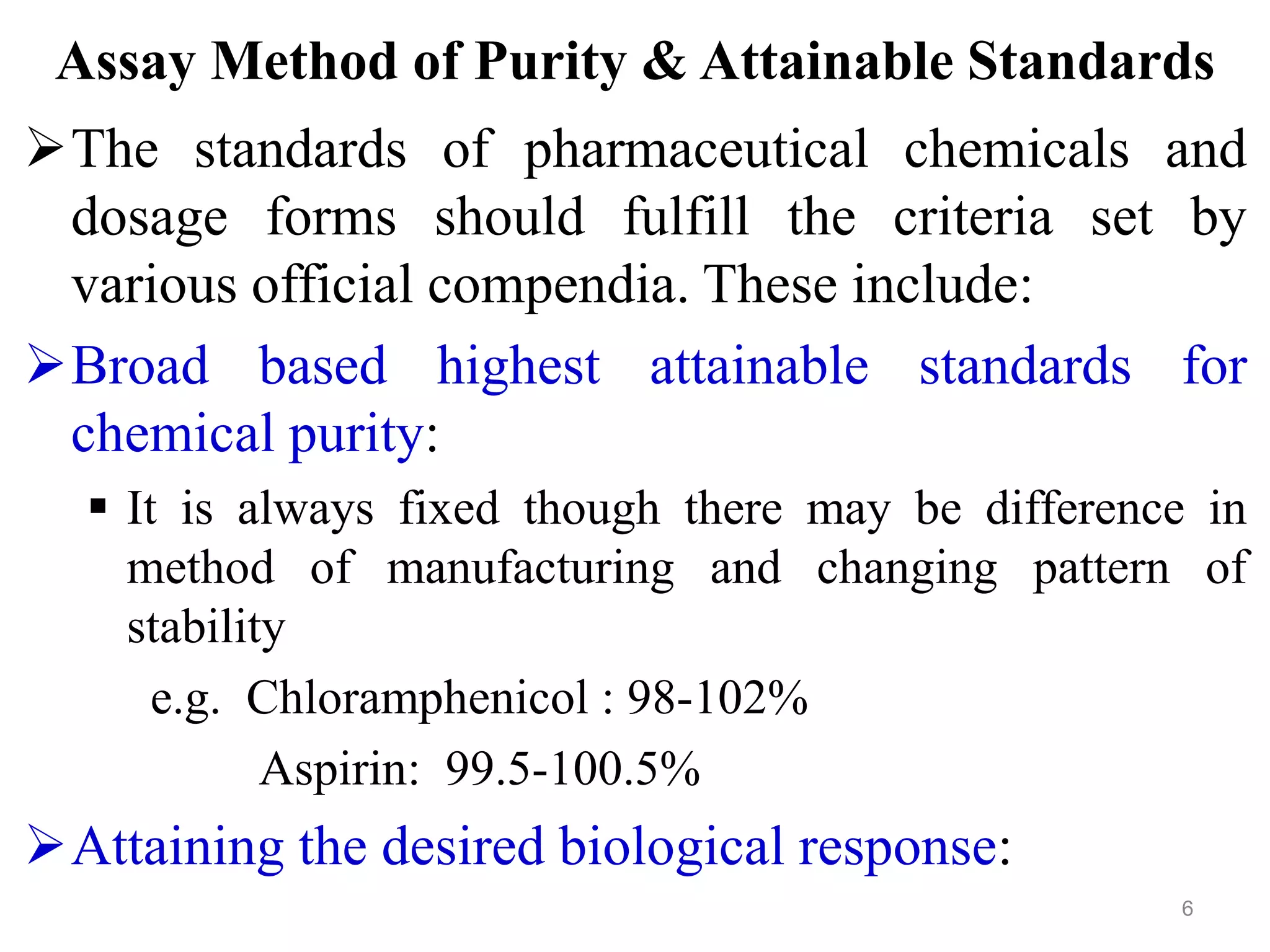
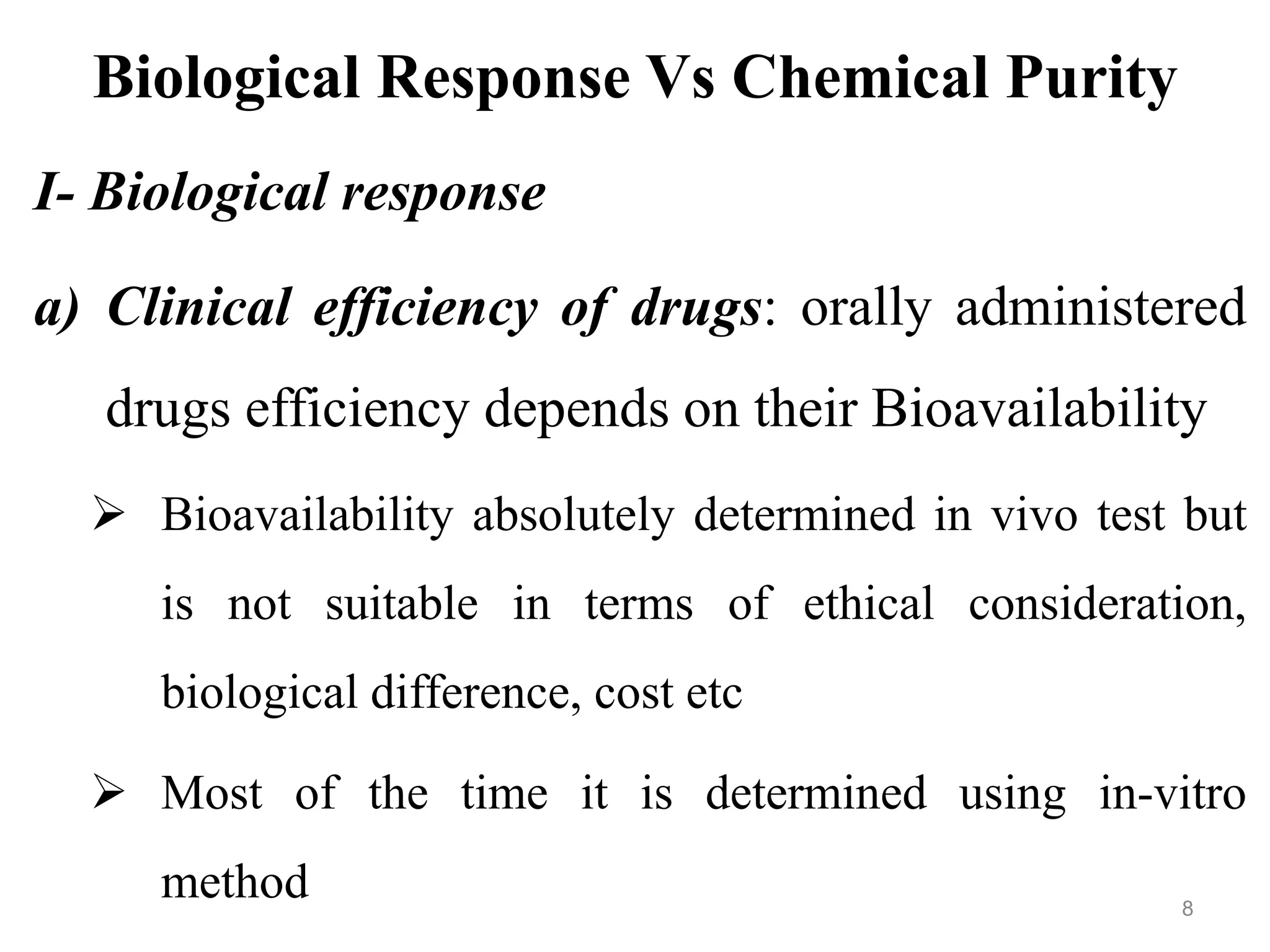
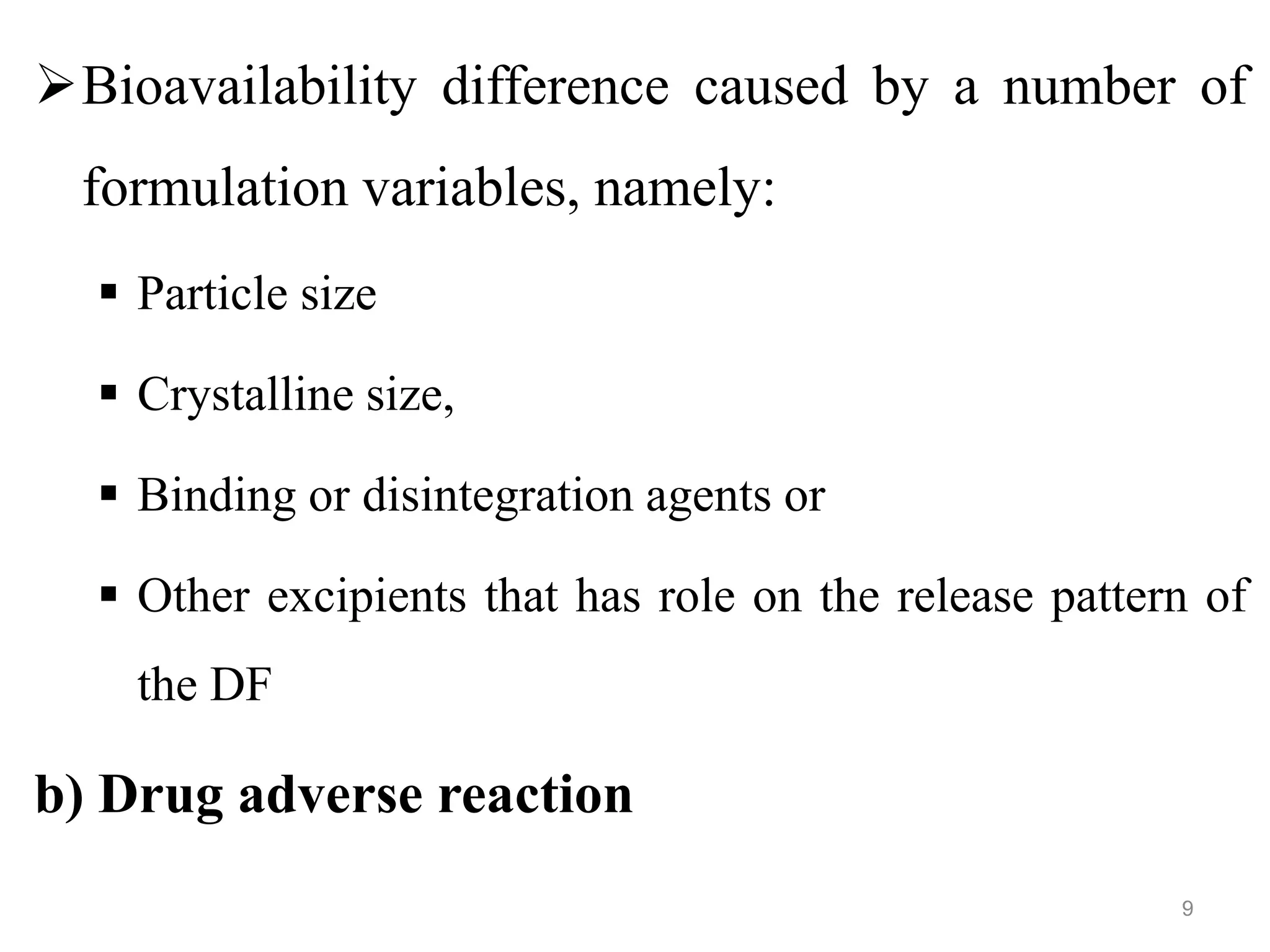
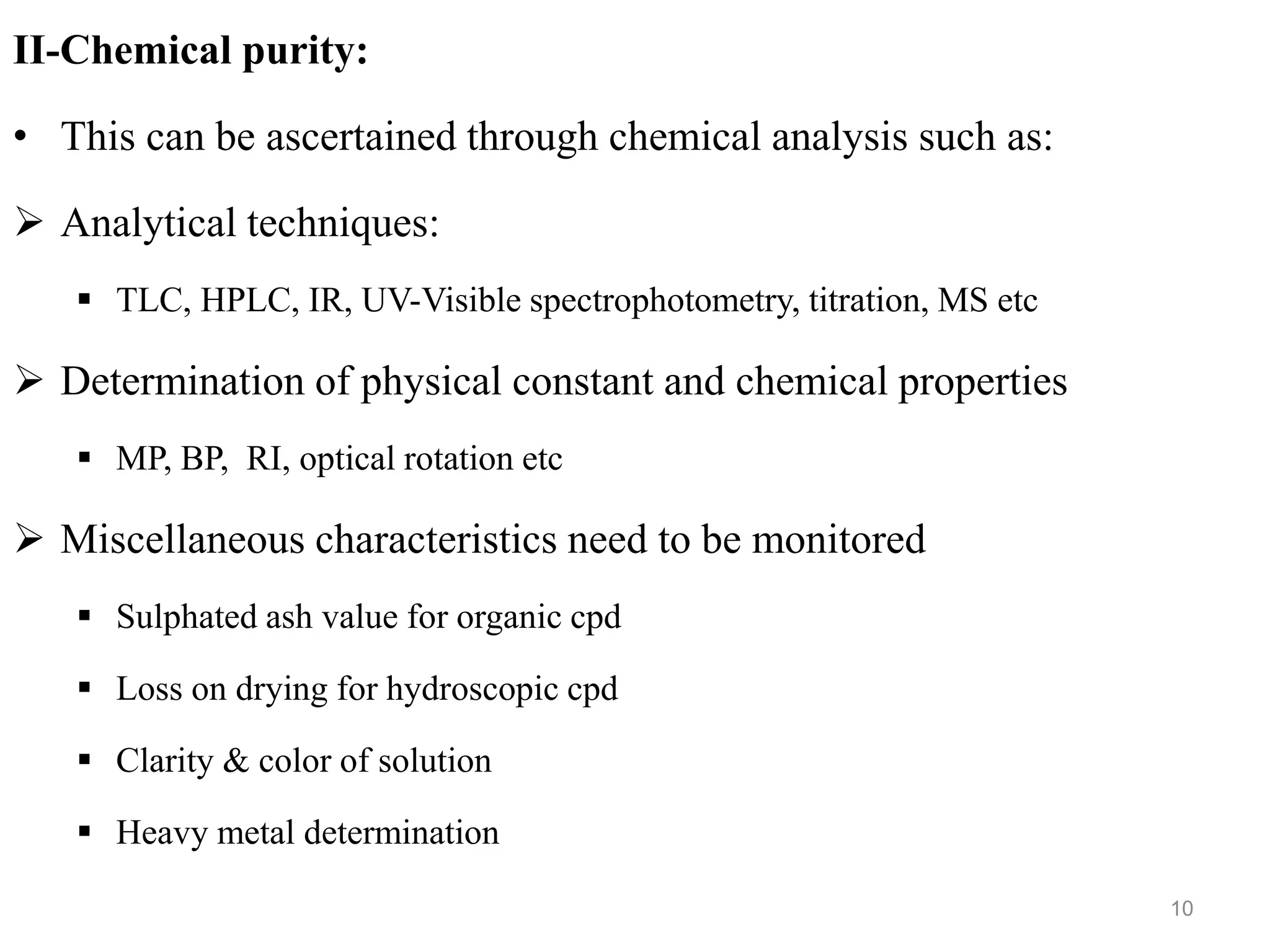
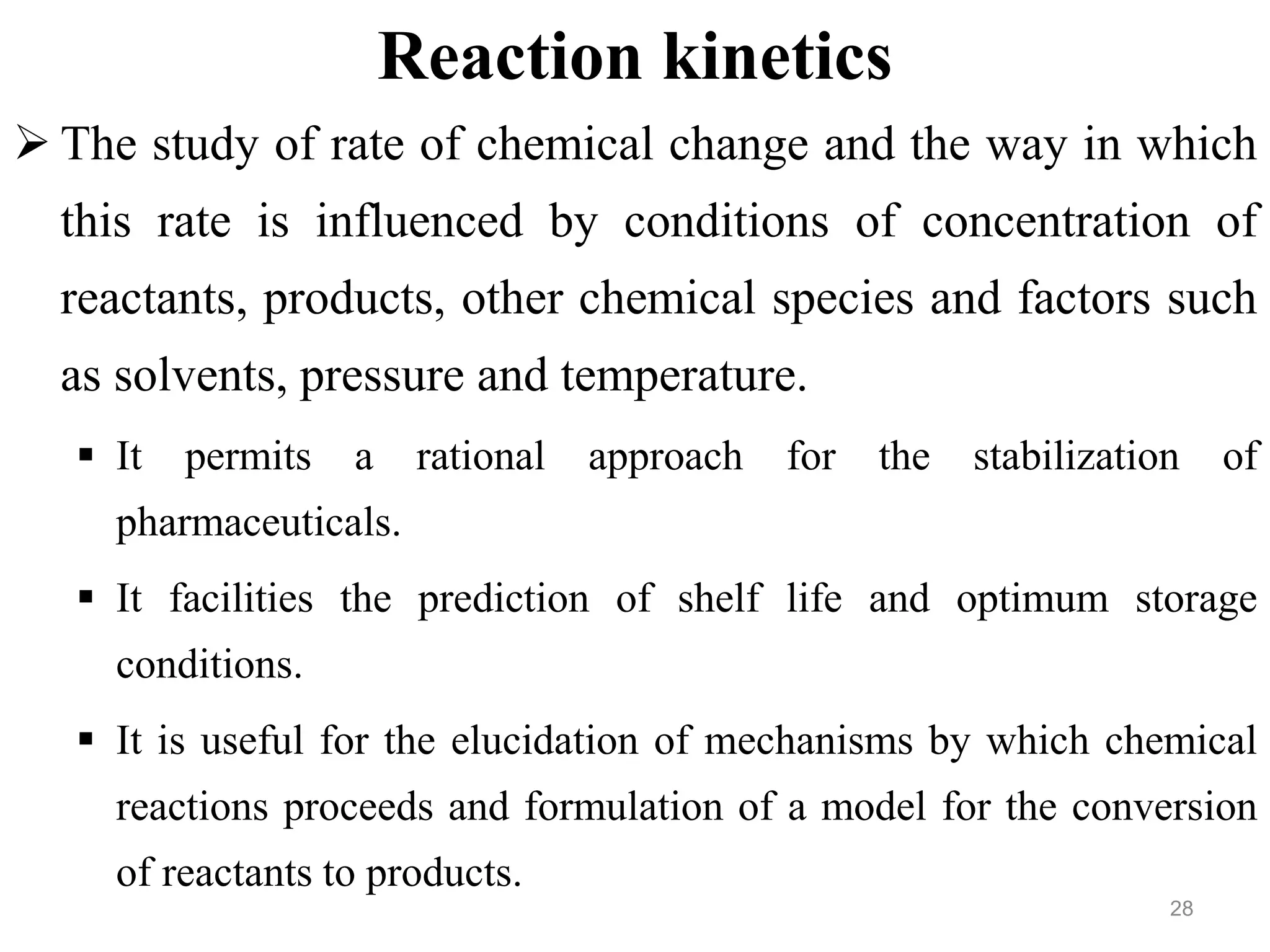
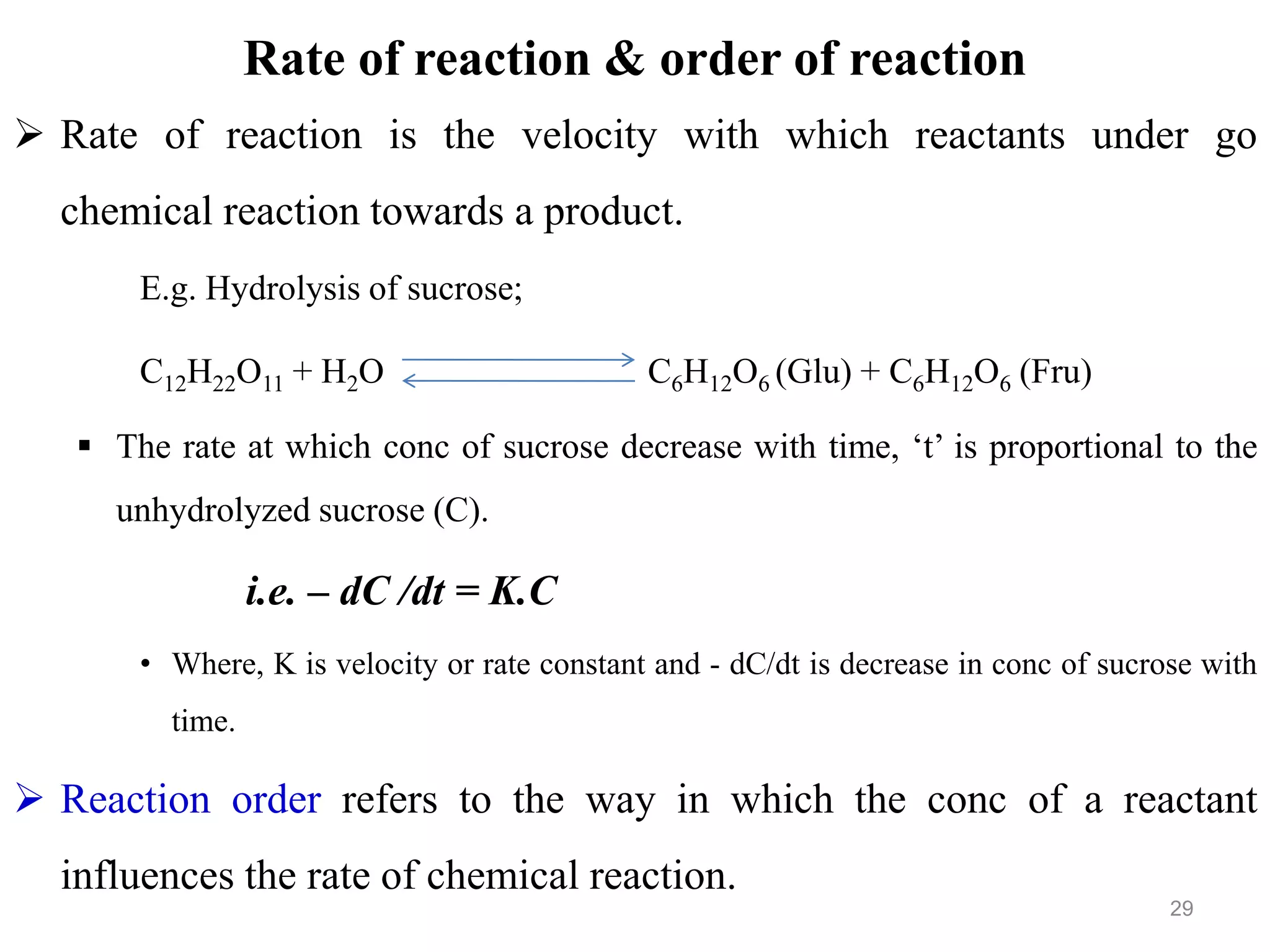
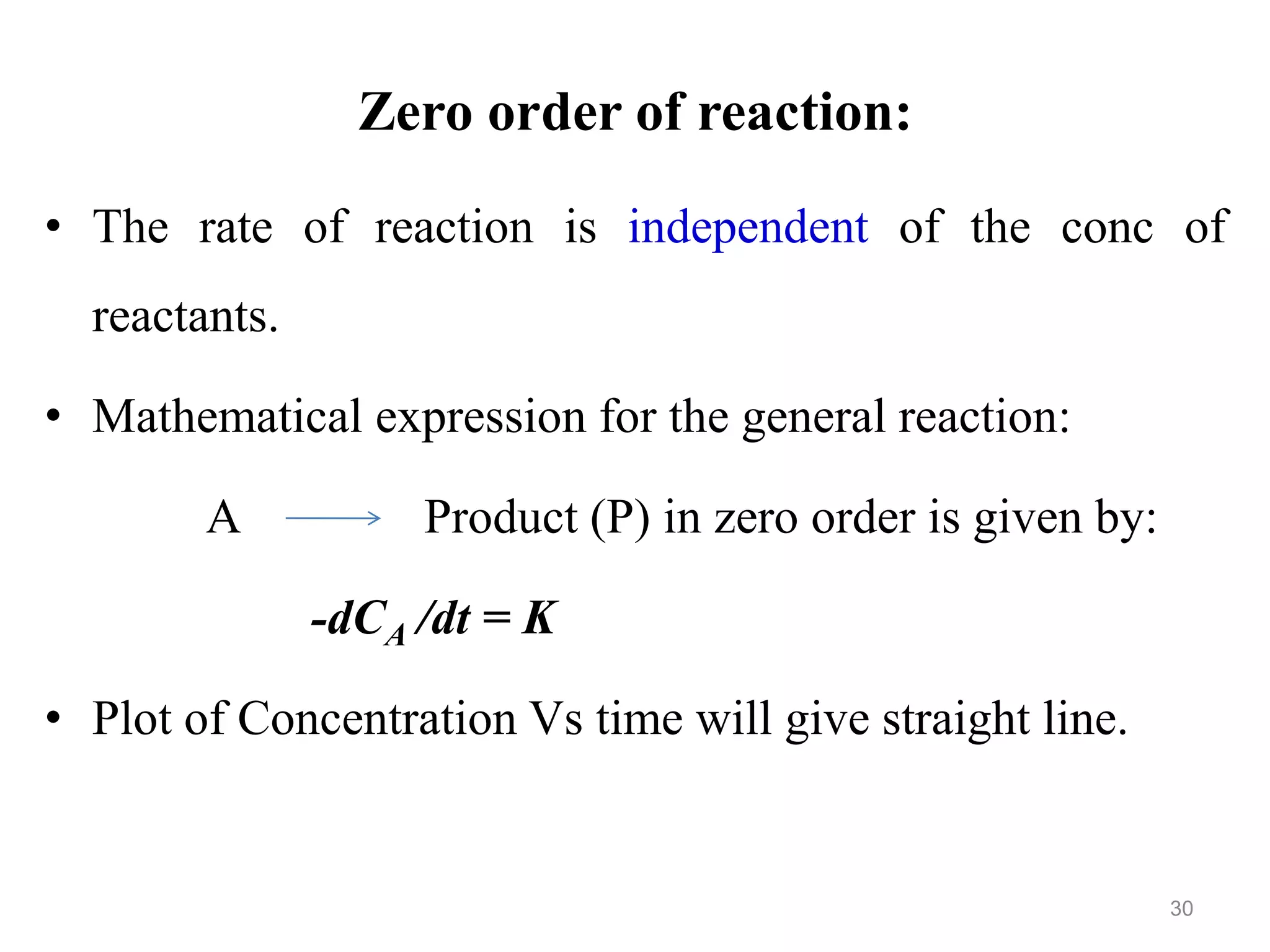
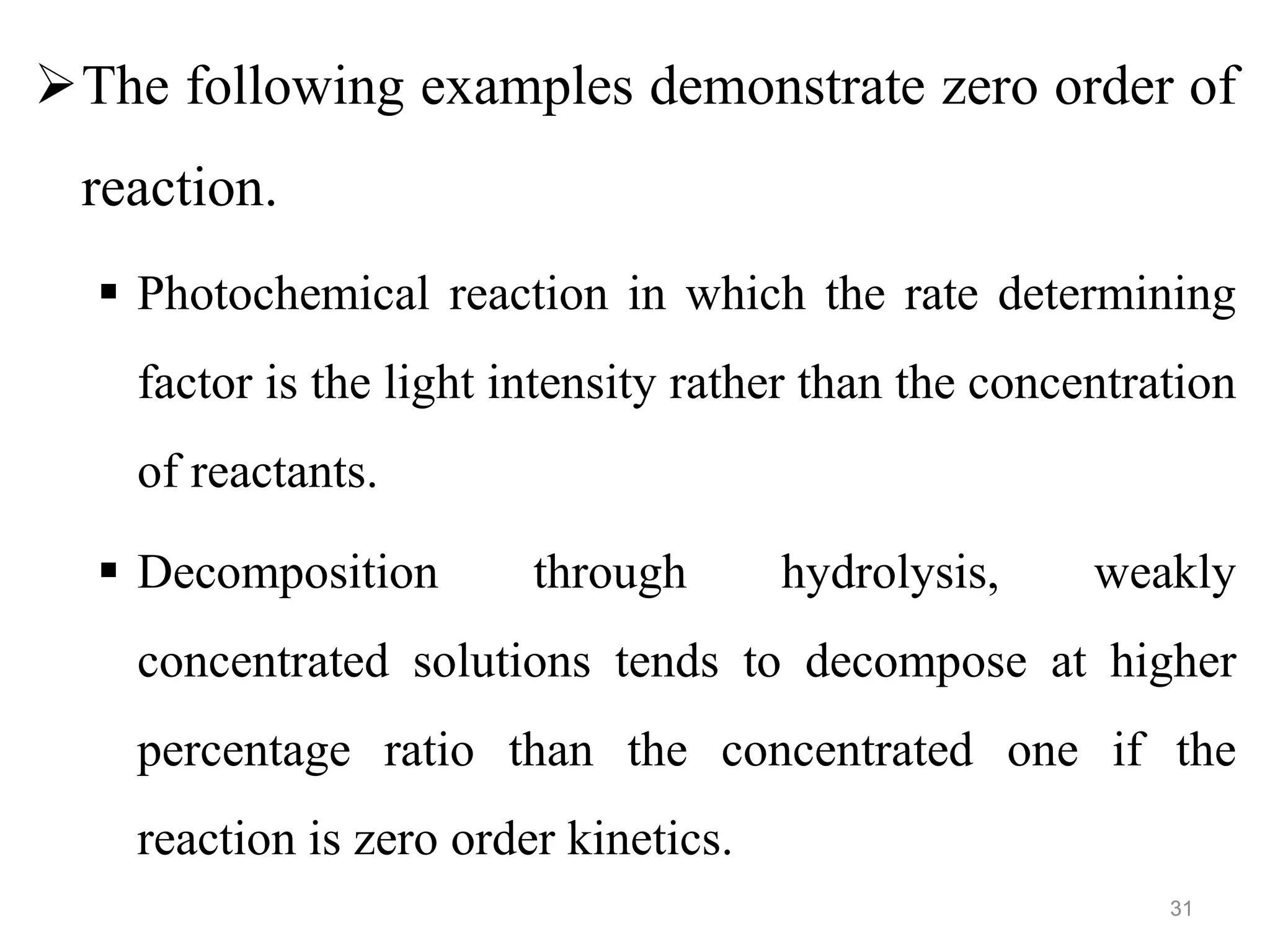
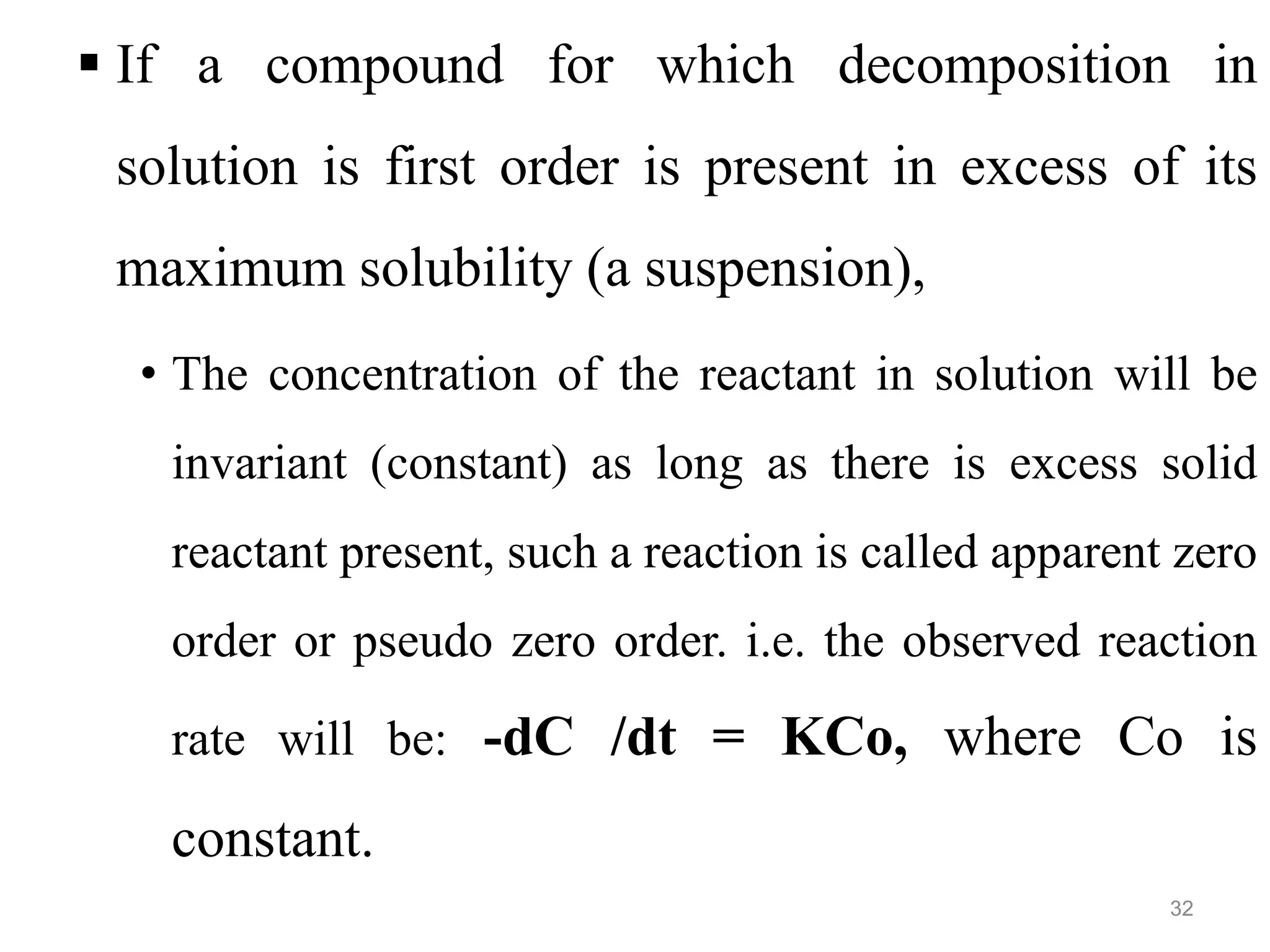
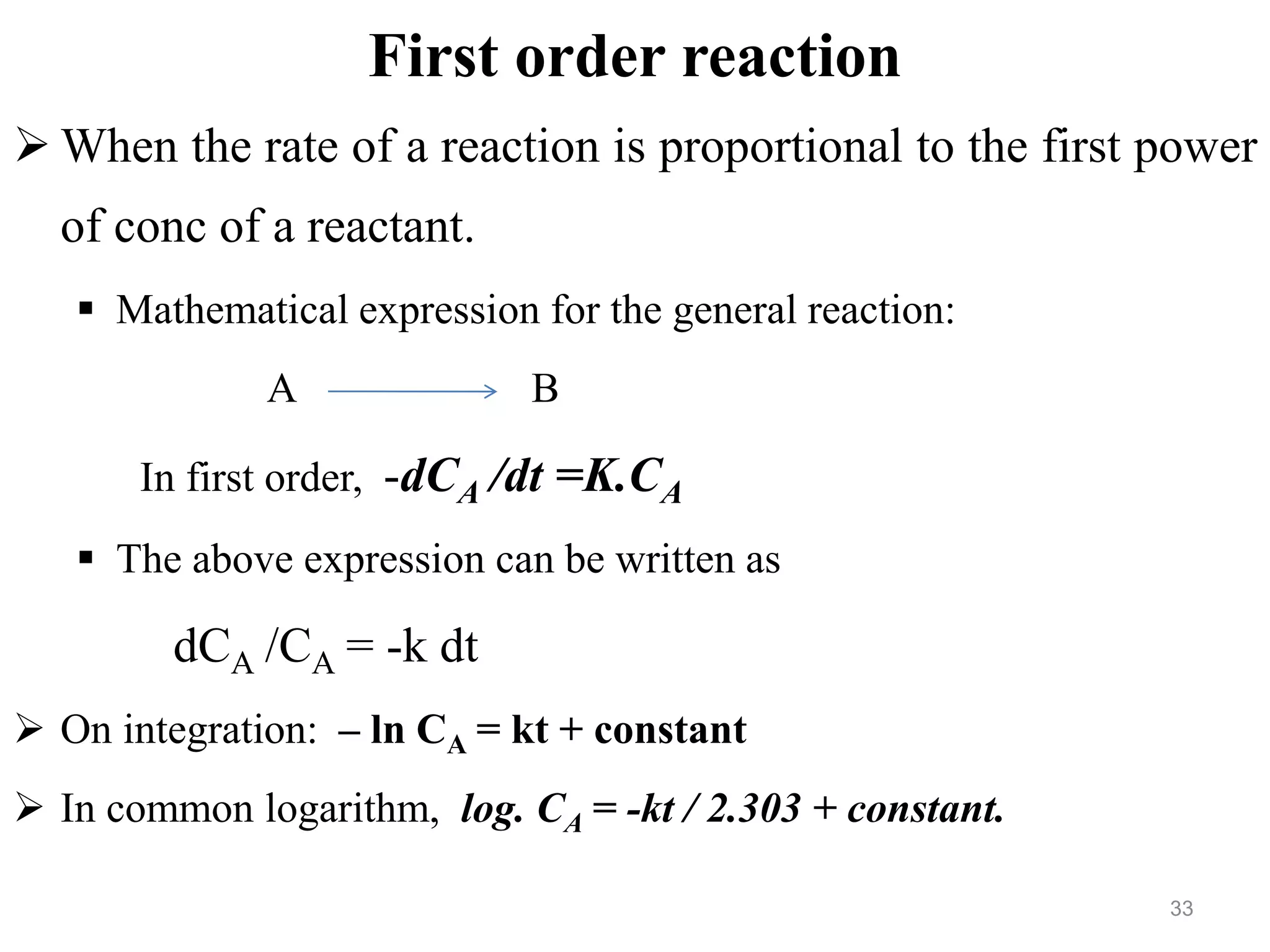
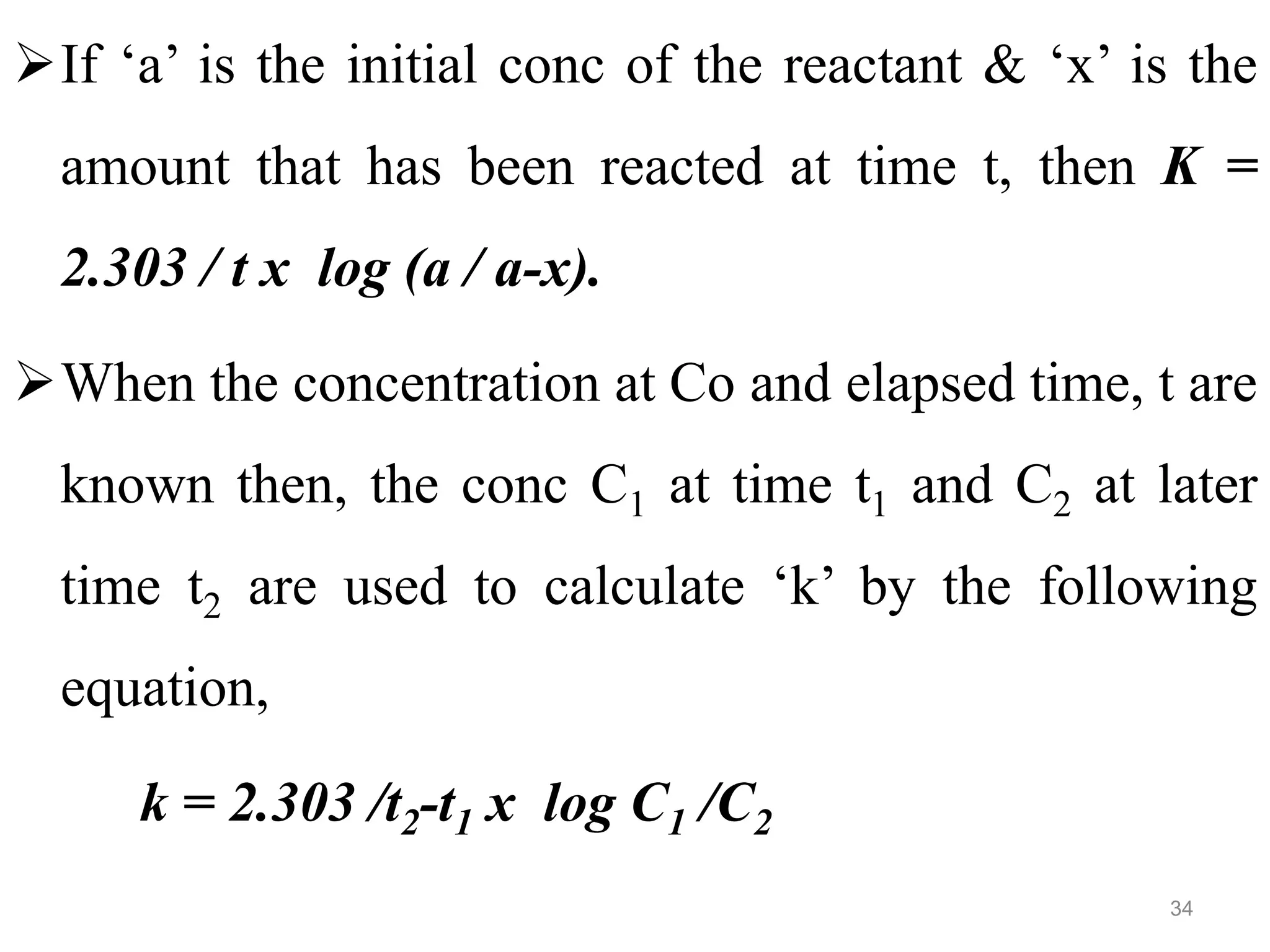
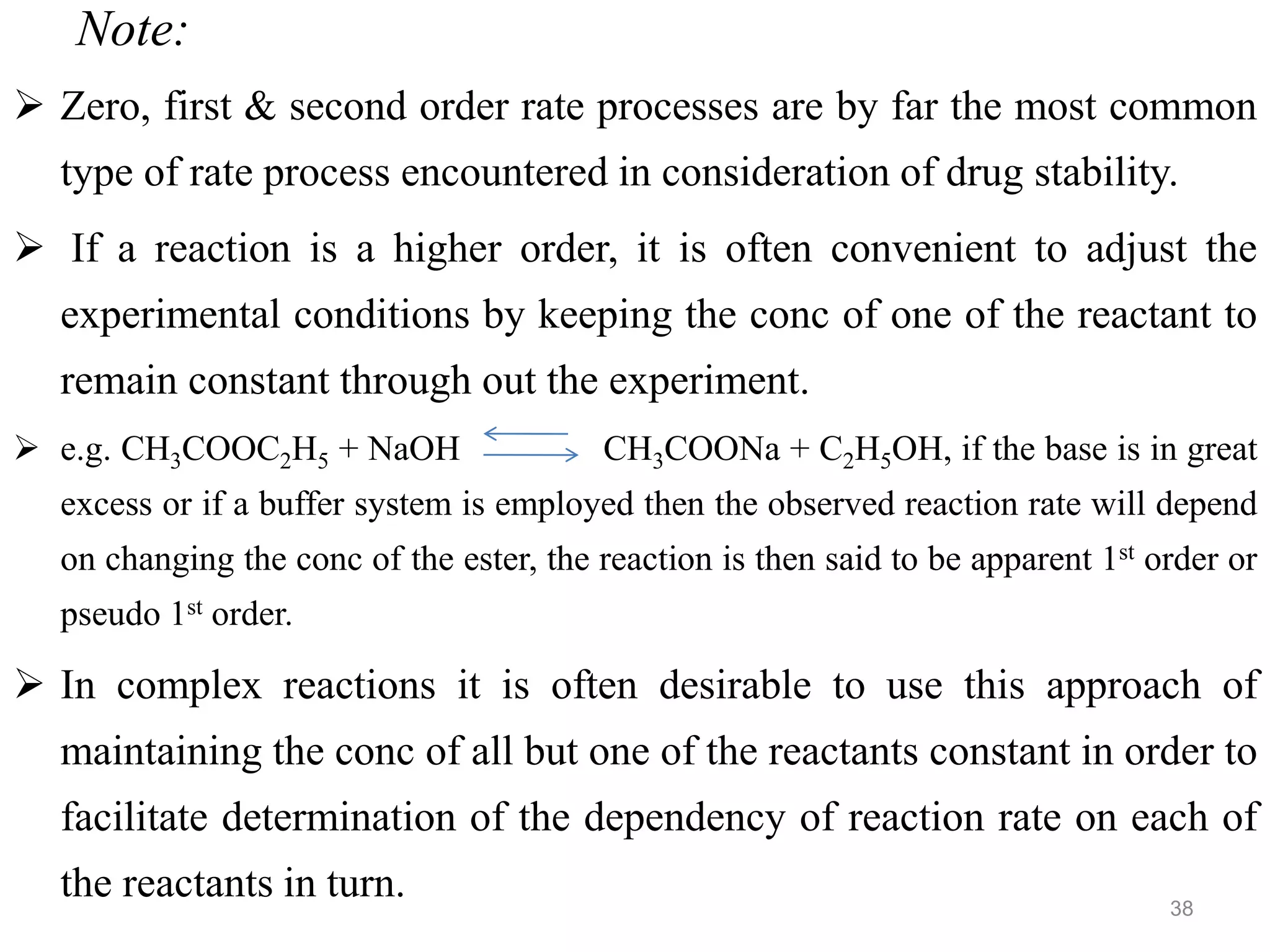
This document discusses purity and factors that can affect the purity of pharmaceutical products. It defines purity as the state where no impurities can be detected by experimental methods. Absolute purity is never achieved in practice. Several parameters like description, identification tests, physical tests, and assays are used to ensure purity according to official compendia. Biological response and chemical purity are also discussed. Various factors that can contribute to instability include incompatibility reactions, oxidation, hydrolysis, decarboxylation, racemization, photochemical degradation, and radiation used for sterilization. Stability indicating studies are important to understand degradation pathways and ensure acceptable potency and safety over a product's shelf life.








![ Many oxidative hydrolytic reactions are catalyzed by both [H+] or
[OH-],
The pH range of minimum decomposition or
Maximum stability depends up on
• The ions having greatest effect on the reaction
In general, [OH-] has stronger catalytic effect & the maximum
stability is often found between pH 3 & 4.
Sometimes it is necessary to compromise b/n the optimum pH for
stability & that of pharmacologic activity,
e.g. several local anesthetics are most stable at acidic pH where as the desired
biological activity is possible at neutral or slightly alkaline conditions.
18](https://image.slidesharecdn.com/06purityimpurity1-220912140750-f1e00c76/75/06Purity-Impurity-1-ppt-18-2048.jpg)

![d) Decarboxylation:
Protolytic solid state degradation through
decarboxylation is less common
Due to high heat of activation requirement to favor
the reaction (25-30 Kcal).
However, first order reaction catalyzed by [H+] at high
temperature can take place for P-aminosalicylic acid to
under go protolytic degradation to M-aminophenol &
CO2.
21](https://image.slidesharecdn.com/06purityimpurity1-220912140750-f1e00c76/75/06Purity-Impurity-1-ppt-21-2048.jpg)



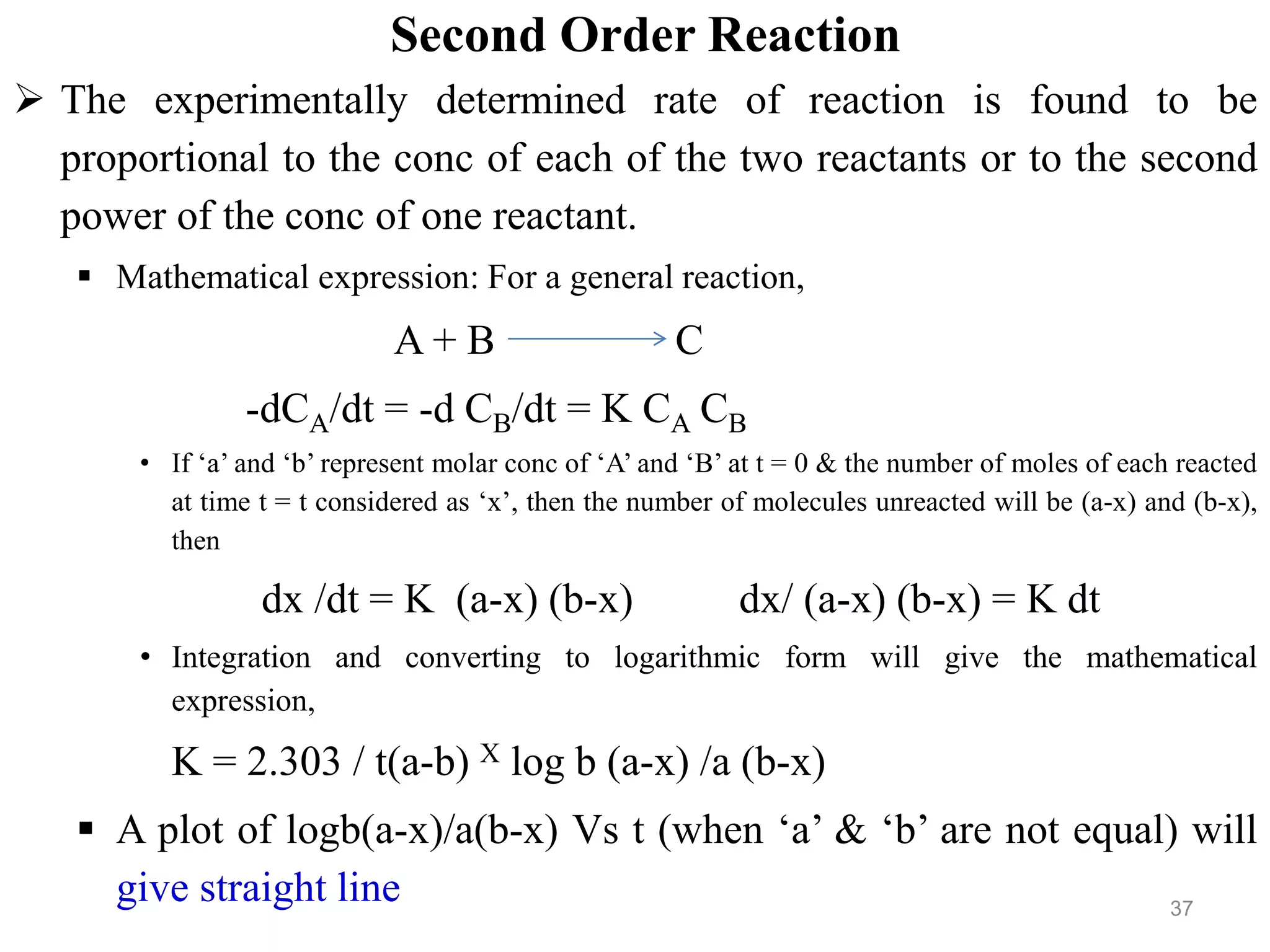
![Third order rate of reaction
The experimentally determined rate of reaction is found to be
proportional to:
The concentration of each of the three reactants.
Or the conc of one of the reactant & the second power of the conc of the other.
Or the third power of the conc of a single reactant.
• Mathematical expression: For a general reaction,
A+B+C D at molar conc of ‘a’, ‘b’ & ‘c’.
Rate equation, -dCA/dt = -dCB/dt = -dCC/dt = KCACBCC
dx /dt = K(a-x) (b-x) (c-x), when a = b = c then,
dx /dt = K (a-x)3, on integration
1 / 2 (a-x)2 = Kt + constant
Evaluating the constant by substituting zero for ‘x’ at t = 0 will give the expression
K = 1 / 2t [1/ (a-x)2 – 1 /a2 ]
39](https://image.slidesharecdn.com/06purityimpurity1-220912140750-f1e00c76/75/06Purity-Impurity-1-ppt-39-2048.jpg)