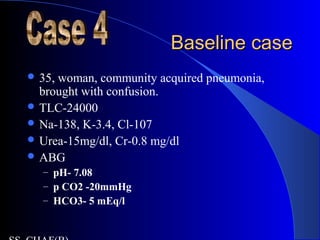
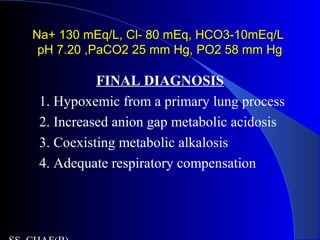
This patient presents with abdominal pain, vomiting, fever and confusion. Arterial blood gas analysis reveals:
- Metabolic acidosis with an increased anion gap of 40, indicating lactic acidosis from sepsis or diabetic ketoacidosis.
- The bicarbonate level is higher than predicted based on the anion gap, suggesting there is also a coexisting metabolic alkalosis present.
- Hypoxemia is seen, and the elevated A-a gradient suggests a primary lung problem like aspiration pneumonia contributing to respiratory compromise in addition to the metabolic derangements.











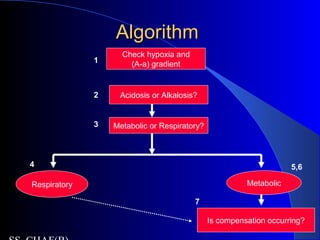


![(A-a gradient)(A-a gradient)
partial pressure of oxygen in the alveolus
(Alveolar gas equation)
– PAO2= FiO2 x (PB- PH2O) – PaCO2/R
– PAO2= 0.21 x (760-47) – PaCO2/0.8
– PAO2= 150-1.25( PACO2)
– PACO2 = PaCO2 (CO2 freely diffuses)
A-a gradient = PAO2 - PaO2
– = [ 150-1.25 (PaCO2) ] - PaO2
– Normal A-a gradient is 10-20 mm Hg](https://image.slidesharecdn.com/abg2014-170402125904/85/Abg-2014-15-320.jpg)



![For metabolic acidosis-For metabolic acidosis-
what is the anion gap?what is the anion gap?
AG = [Na+] - ([Cl-
] + [HCO3-
])
normal anion gap is 12
Unmeasured Anions Unmeasured Cations
Proteins, mostly albumin 15 mEq/L Calcium 5 mEq/L
Organic acids 5 mEq/L Potassium 4.5 mEq/L
Phosphates 2 mEq/L Magnesium 1.5 mEq/L
Sulfates 1 mEq/L
Totals: 23 mEq/L 11 mEq/L
Anion Gap = GAP between unmeasured anions and cations.](https://image.slidesharecdn.com/abg2014-170402125904/85/Abg-2014-23-320.jpg)






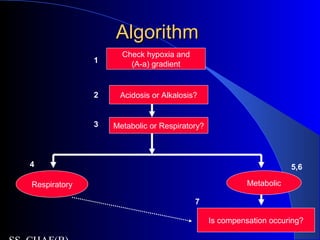
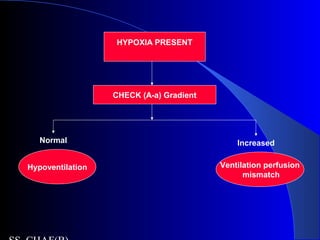
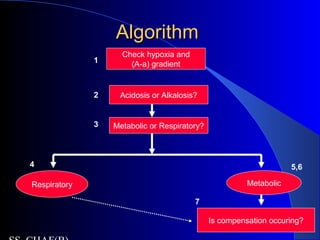
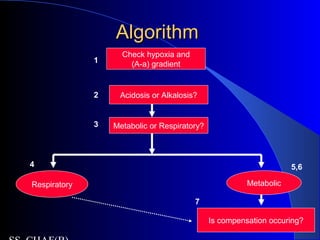
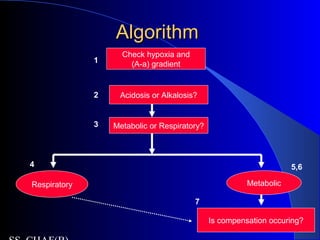
![Is the respiratory system compensatingIs the respiratory system compensating
adequately for a metabolic disturbance?adequately for a metabolic disturbance?
Mainly necessary if disturbance is primarily
metabolic.
Metabolic acidosis
– Expected PaCO2 = [1.5 x serum HCO3-] + 8 + 2.
(Winter’s formula)
– E.g If HCO3 = 10
– Paco2= 21-25
– If <21- then coexisatnt resp alkalosis
– If >25, coexistant resp acidosis](https://image.slidesharecdn.com/abg2014-170402125904/85/Abg-2014-31-320.jpg)



![Na+ 130 mEq/L, Cl- 80 mEq, HCO3-10mEq/LNa+ 130 mEq/L, Cl- 80 mEq, HCO3-10mEq/L
pH 7.20 ,PaCO2 25 mm Hg, PO2 68 mm HgpH 7.20 ,PaCO2 25 mm Hg, PO2 68 mm Hg
1. Assess oxygenation
PaO2 = 58 mm Hg
He has hypoxia
(A-a) gradient= [ 150 - 1.25 (PaCO2) ] - PaO2
= [150 - 1.25 (25)] - 58 = 61
there is a primary lung problem
Possibly aspiration pneumonia](https://image.slidesharecdn.com/abg2014-170402125904/85/Abg-2014-36-320.jpg)






![Na+ 138 mEq/L, Cl- 100 mEq, HCO3-26mEq/LNa+ 138 mEq/L, Cl- 100 mEq, HCO3-26mEq/L
pH 7.08 ,PaCO2 80 mm Hg, PO2 37 mm HgpH 7.08 ,PaCO2 80 mm Hg, PO2 37 mm Hg
1. Assess oxygenation
Is the patient hypoxic?
YES
What is his (A-a) gradient?
A-a gradient = [ 150 - 1.25 (PaCO2) ] - PaO2
[150 - 1.25 (80)] - 37 = 10.
Hypoxia solely due to hypoventilation](https://image.slidesharecdn.com/abg2014-170402125904/85/Abg-2014-44-320.jpg)





![Na+ 135, Cl- 93, HCO3- 30Na+ 135, Cl- 93, HCO3- 30
pH 7.21 / P CO2 75 / pO2 41pH 7.21 / P CO2 75 / pO2 41
1. Assess oxygenation
Hypoxemia present
A-a gradient = [ 150 - 1.25 (PaCO2) ] - PaO2
[150 - 1.25 (75)] - 41 = 15
Hypoxemia is due to
hypoventilation](https://image.slidesharecdn.com/abg2014-170402125904/85/Abg-2014-50-320.jpg)