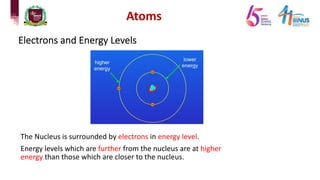
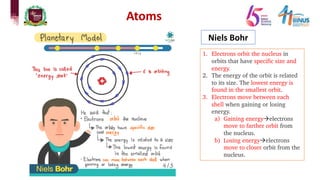
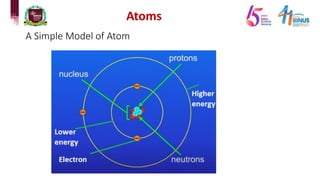
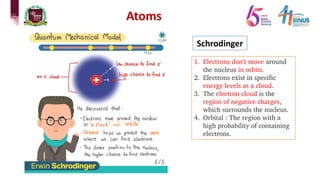
The document discusses atomic models and nuclear physics. It provides information on:
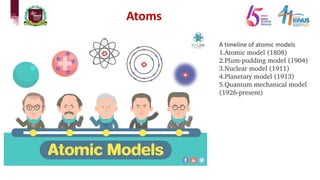
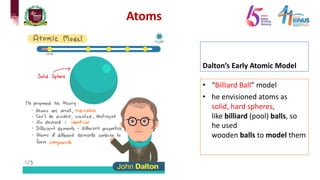
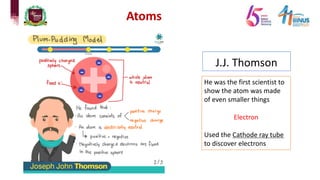
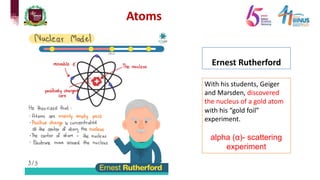
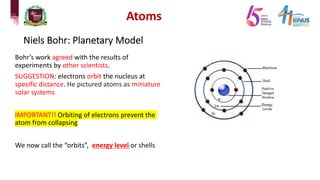
1) Early atomic models including Dalton's billiard ball model, Thomson's plum pudding model, Rutherford's nuclear model, and Bohr's planetary model.
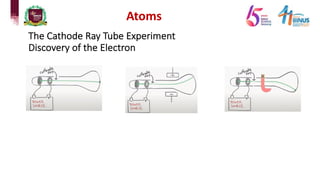
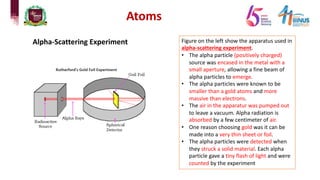
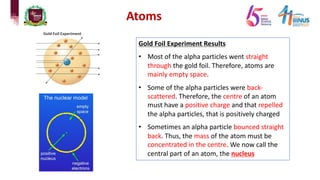
2) Experiments that led to discoveries about the structure of the atom including Thomson's cathode ray tube experiment, Rutherford's gold foil experiment, and Bohr's model of electron orbits.
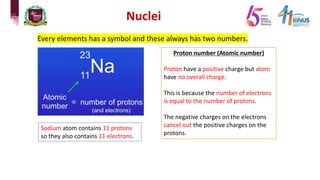
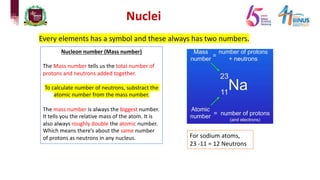
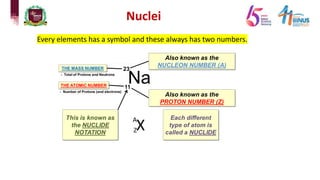
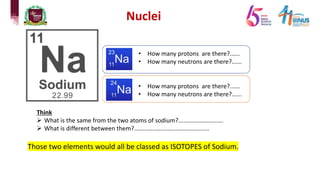
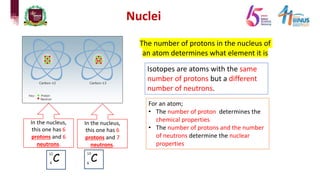
3) Components of the nucleus including protons, neutrons, and isotopes.
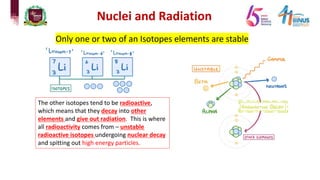
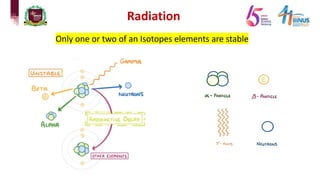
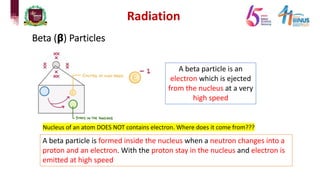
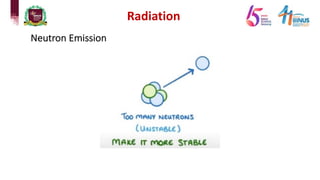
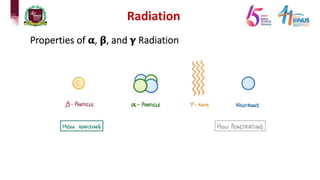
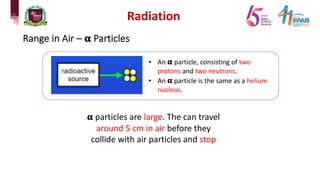
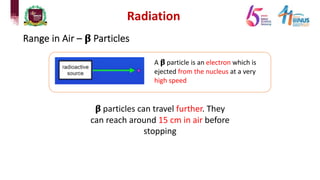
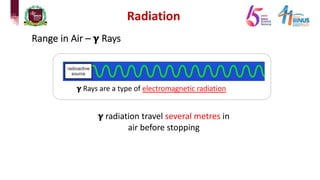
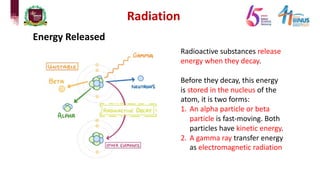
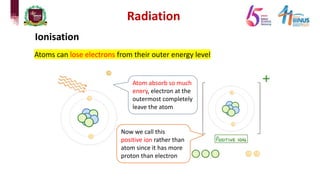
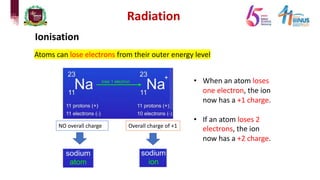
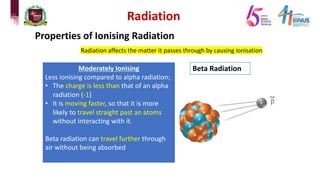
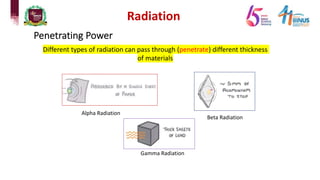
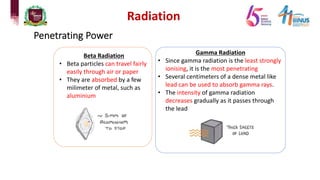
4) Types of radiation including alpha, beta, gamma particles and their properties such as mass, charge, penetration and ionization.
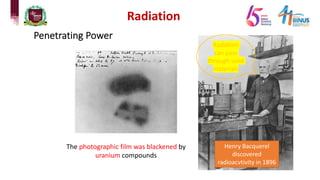
5) Experiments that helped discover radiation and nuclear decay processes.