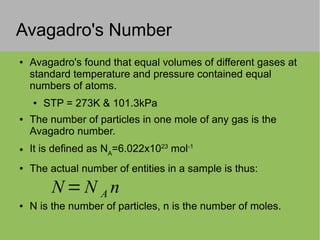
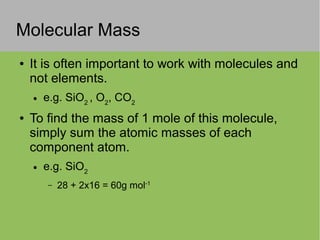
This document discusses key concepts in thermodynamics including:
1) All objects have internal energy due to the vibration and interactions of their internal particles; internal energy can be transferred as thermal energy.
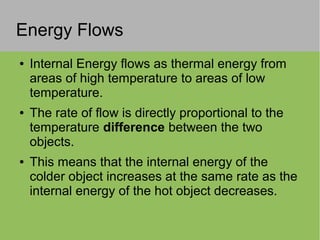
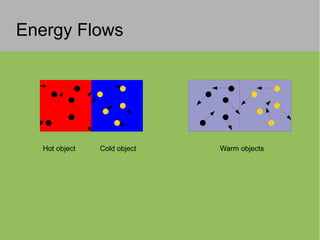
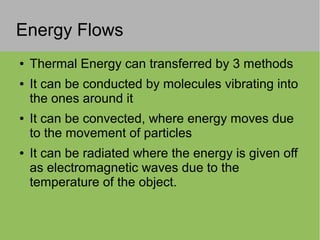
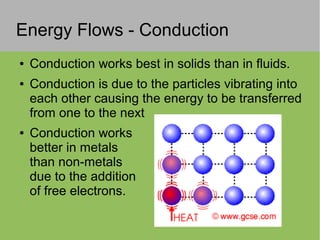
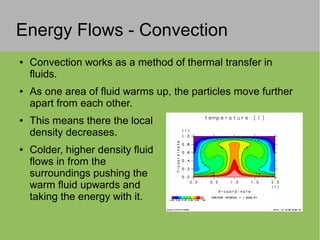
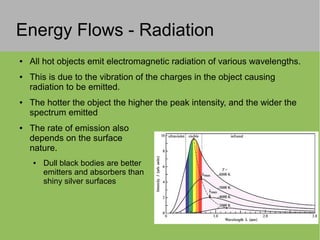
2) Thermal energy flows spontaneously from hot to cold objects and can be transferred by conduction, convection, or radiation.
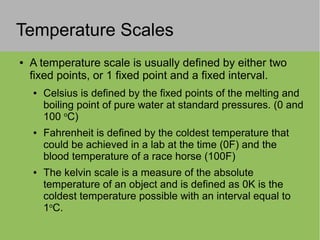
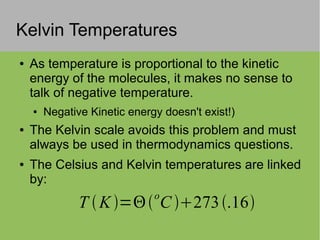
3) Temperature is a measure of average kinetic energy and different temperature scales (Celsius, Fahrenheit, Kelvin) are defined based on fixed points.