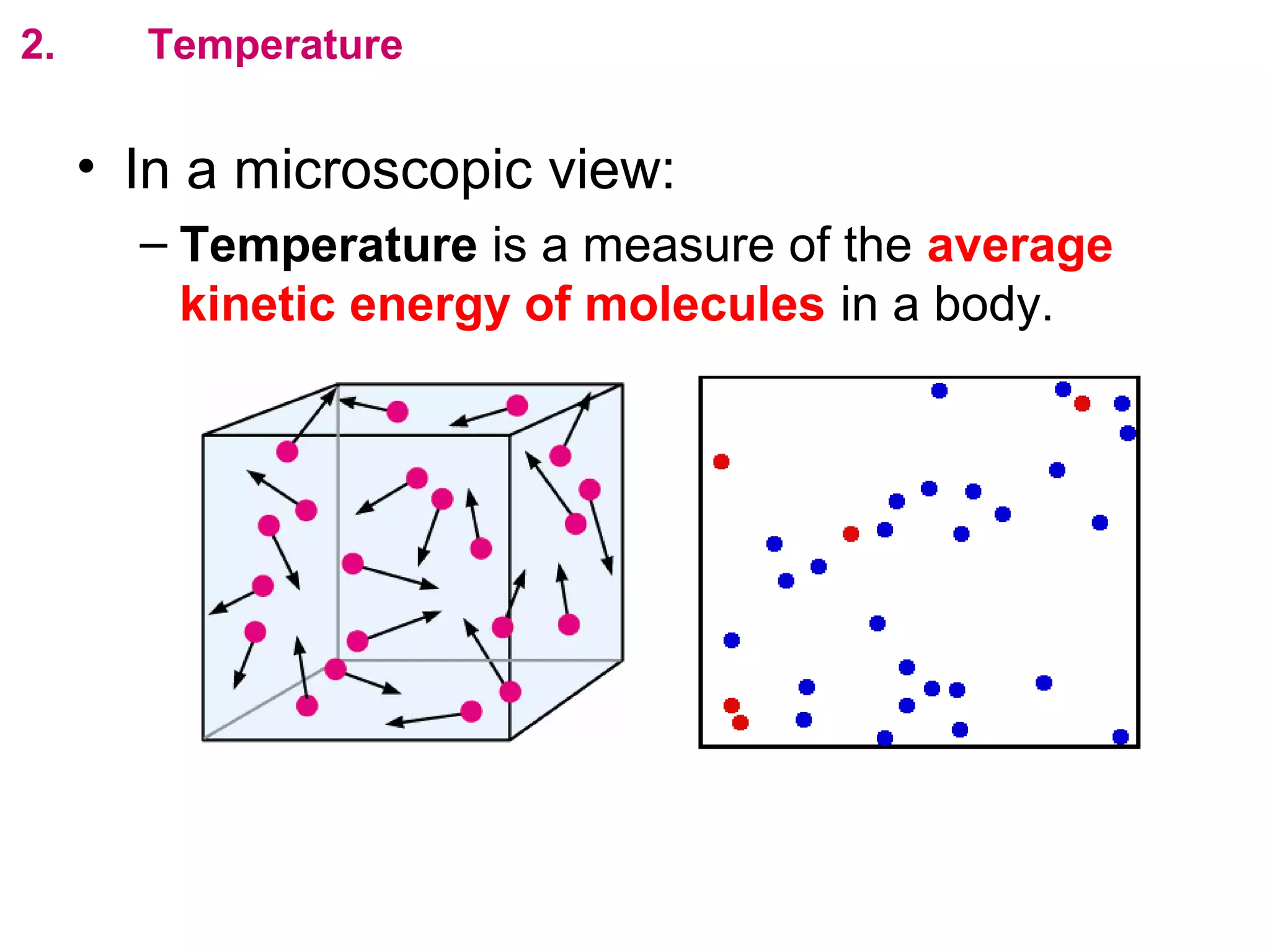
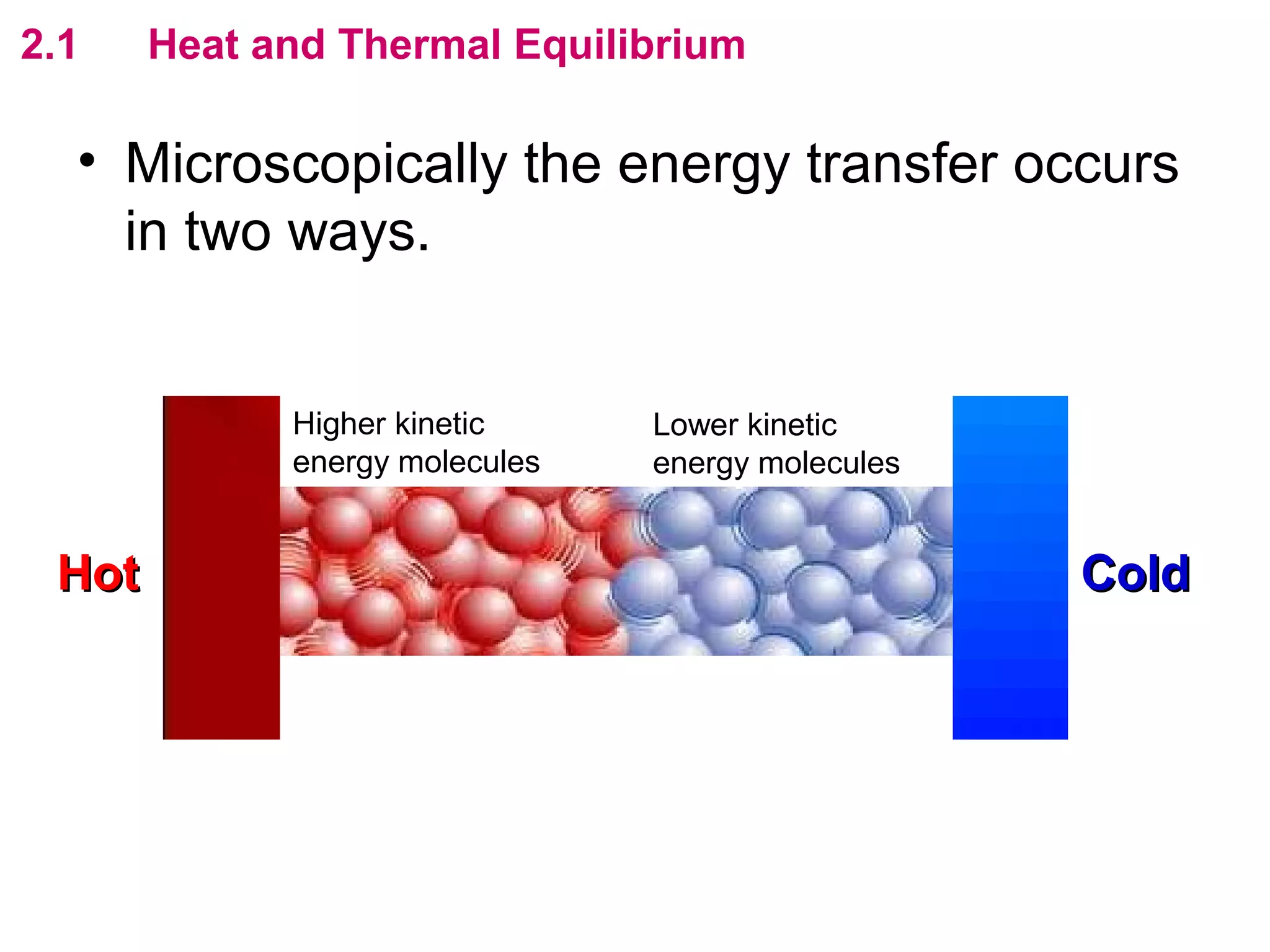
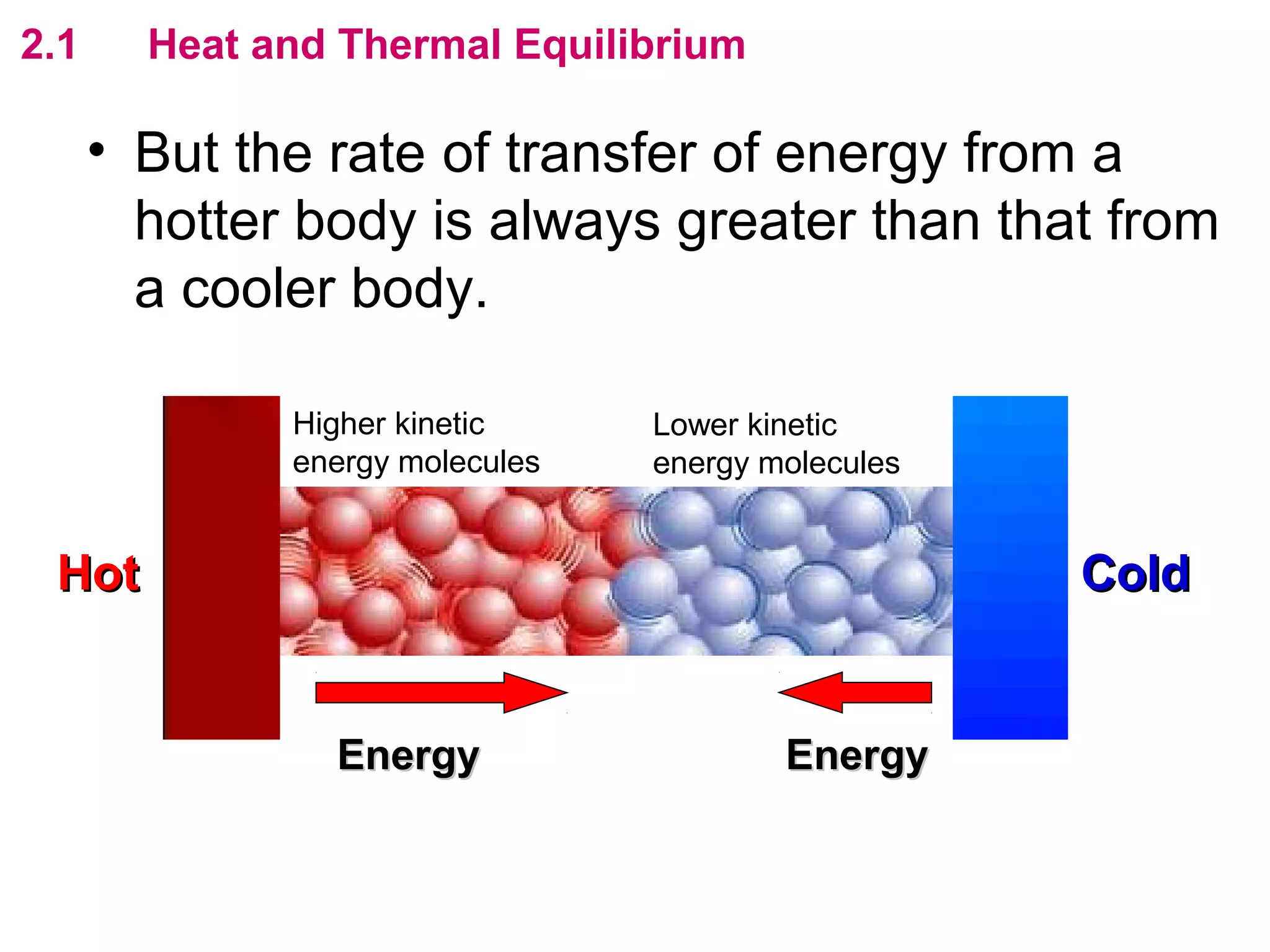
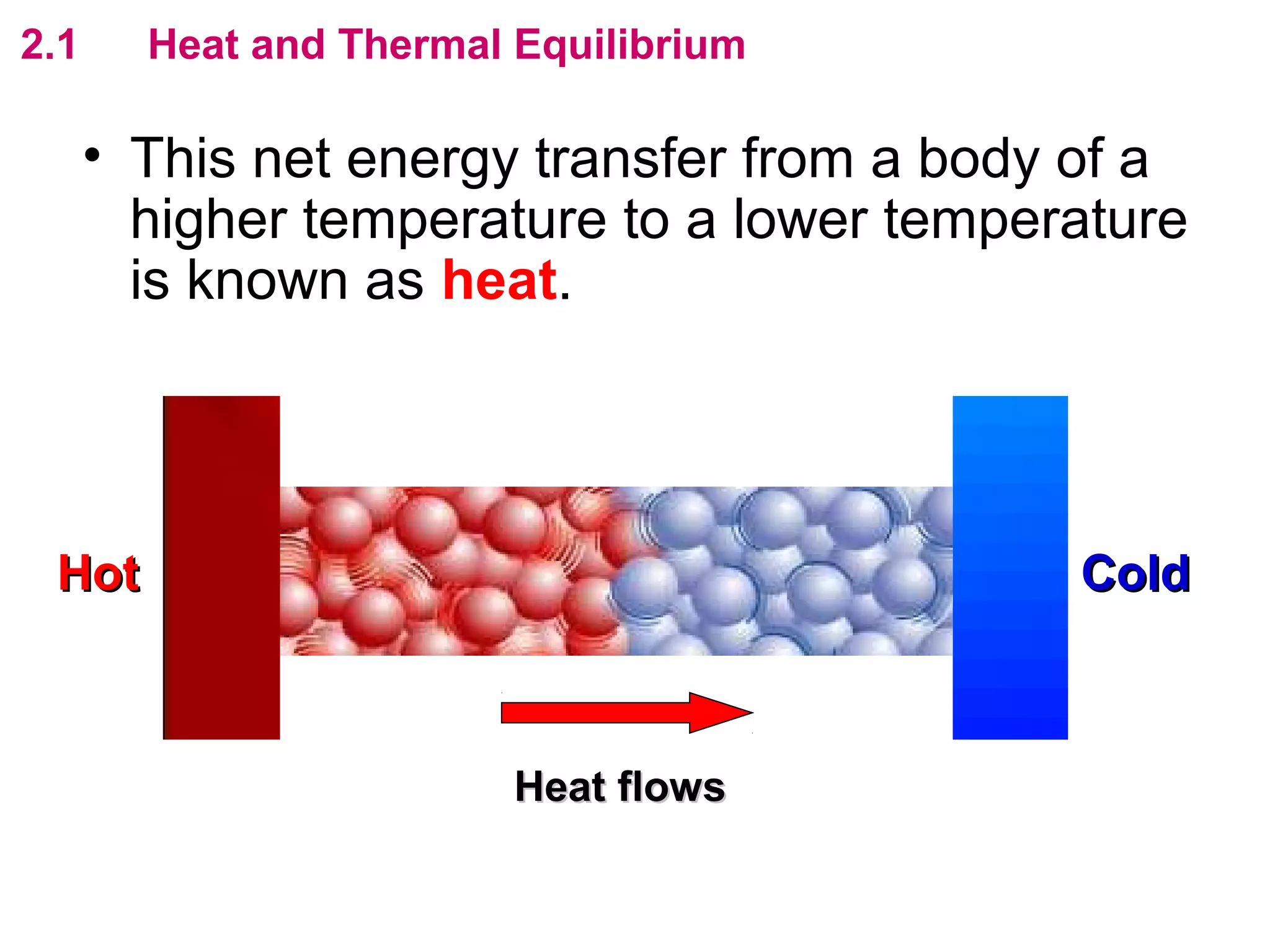
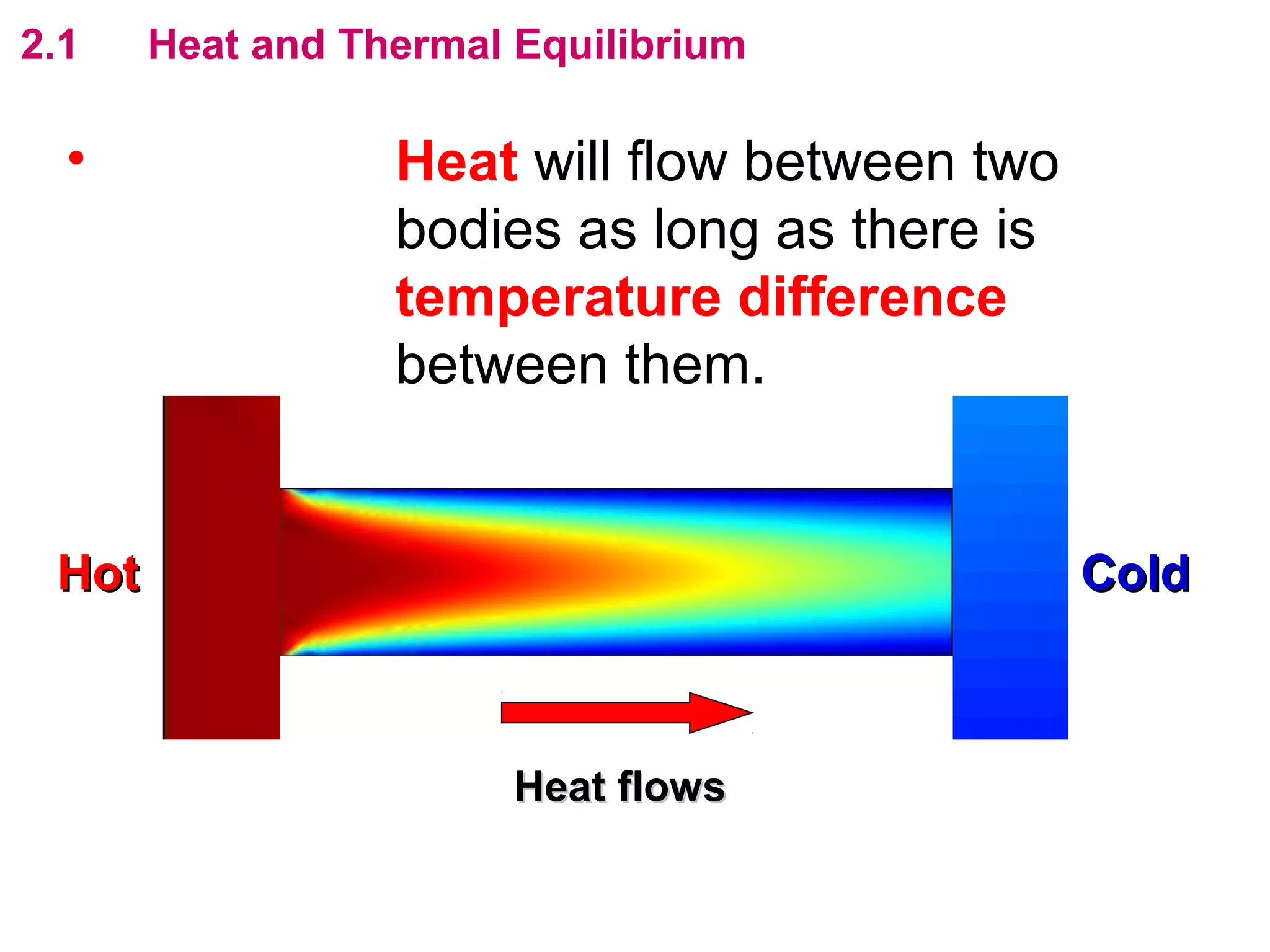
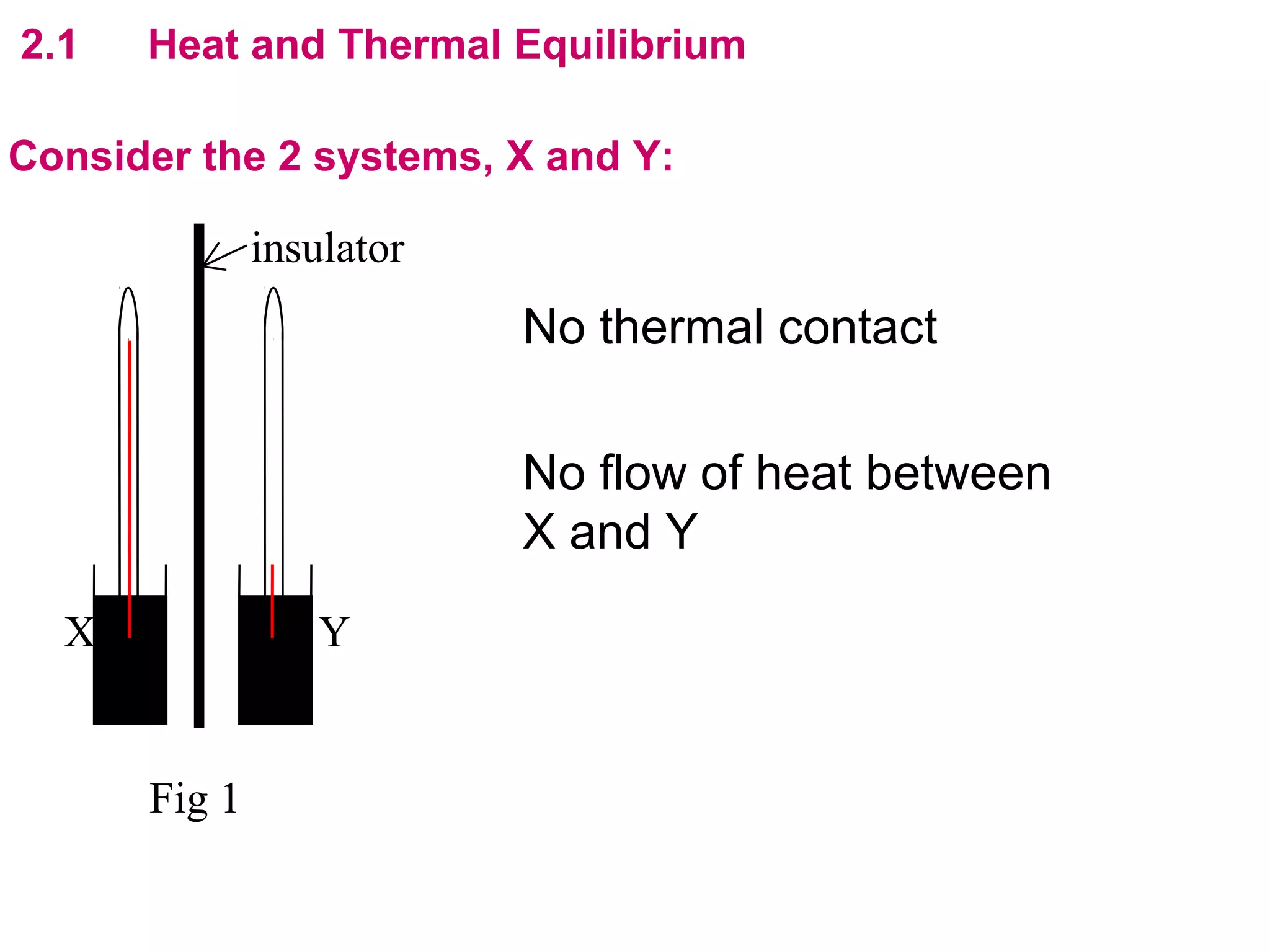
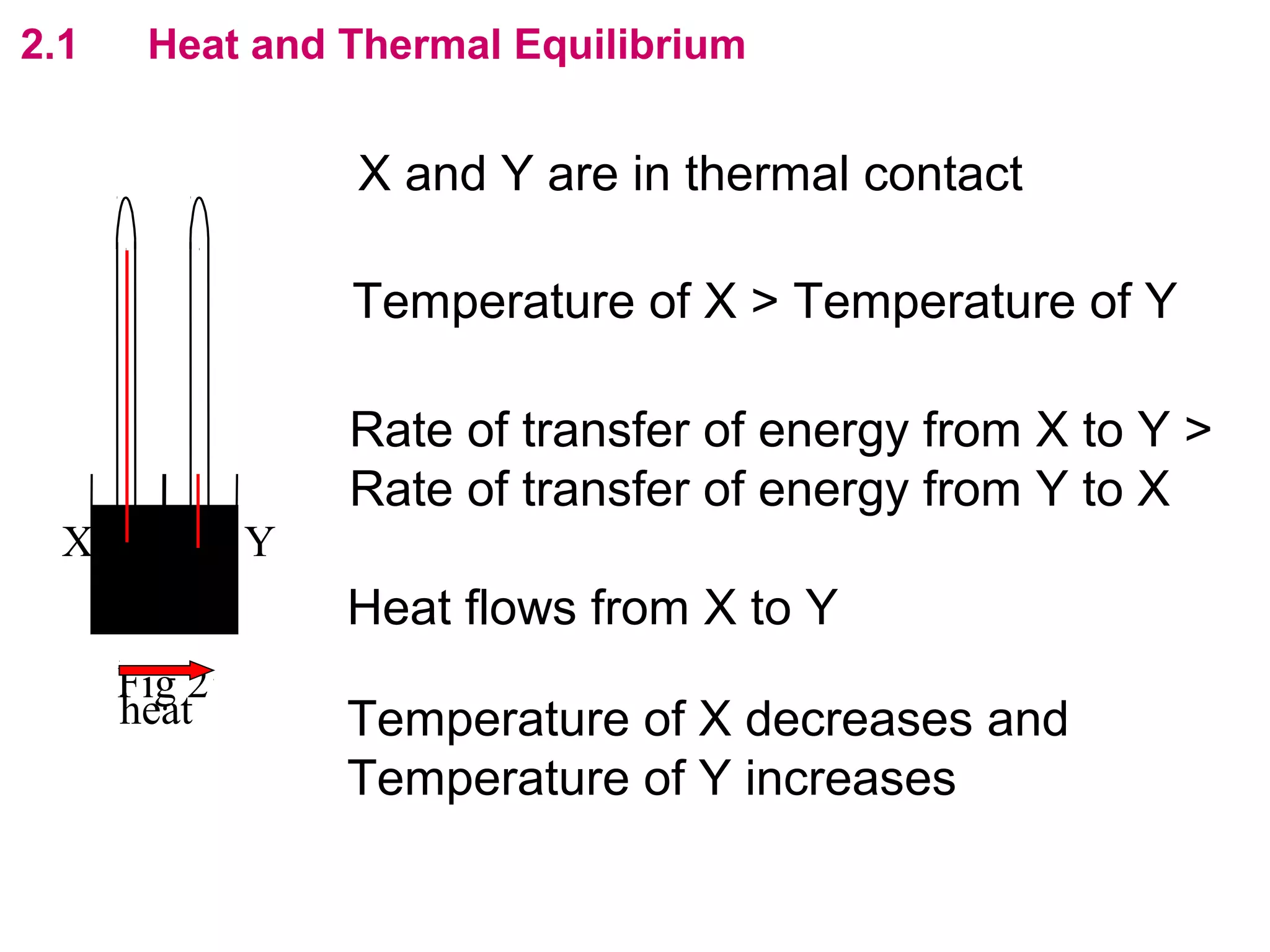
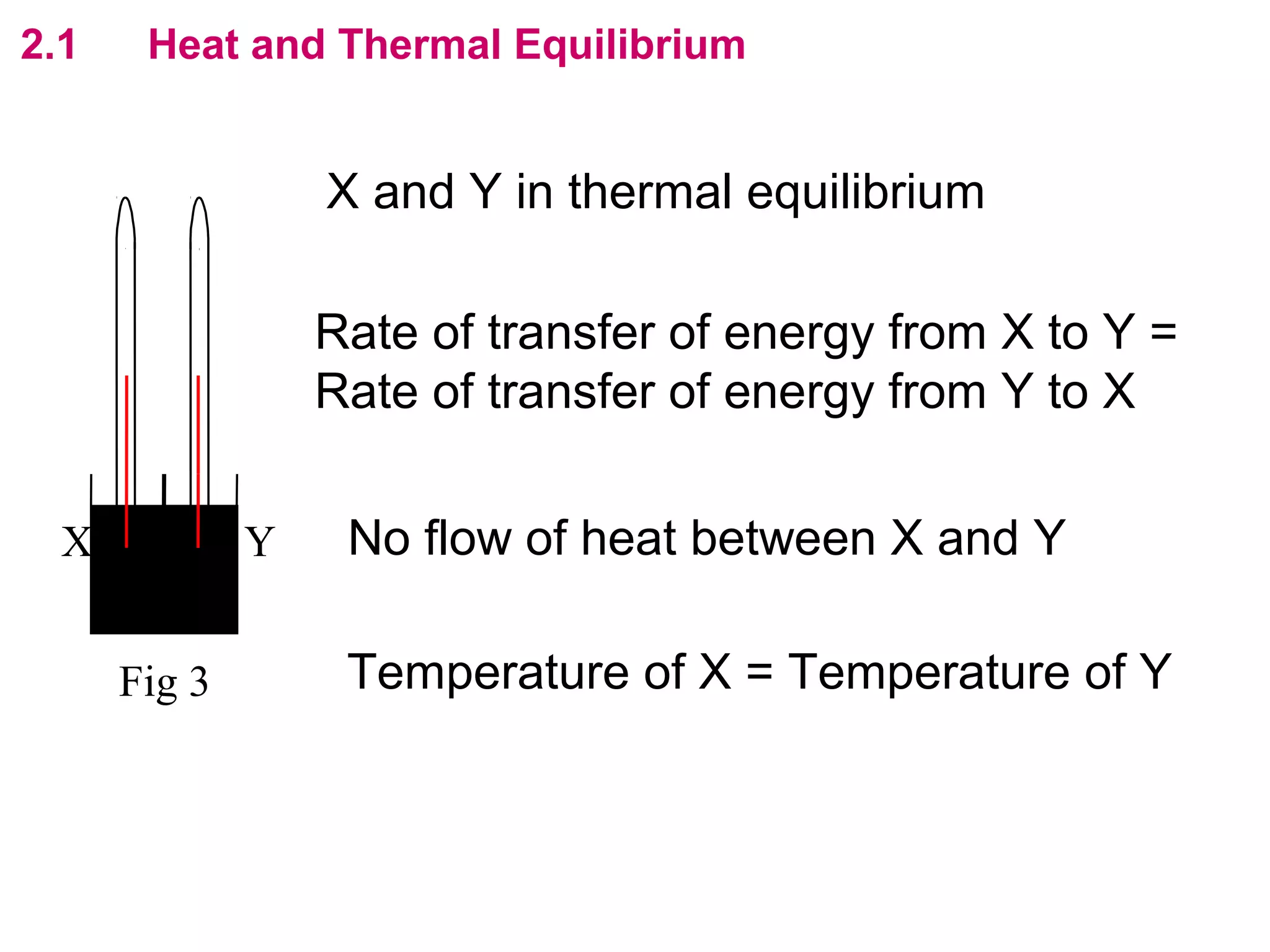
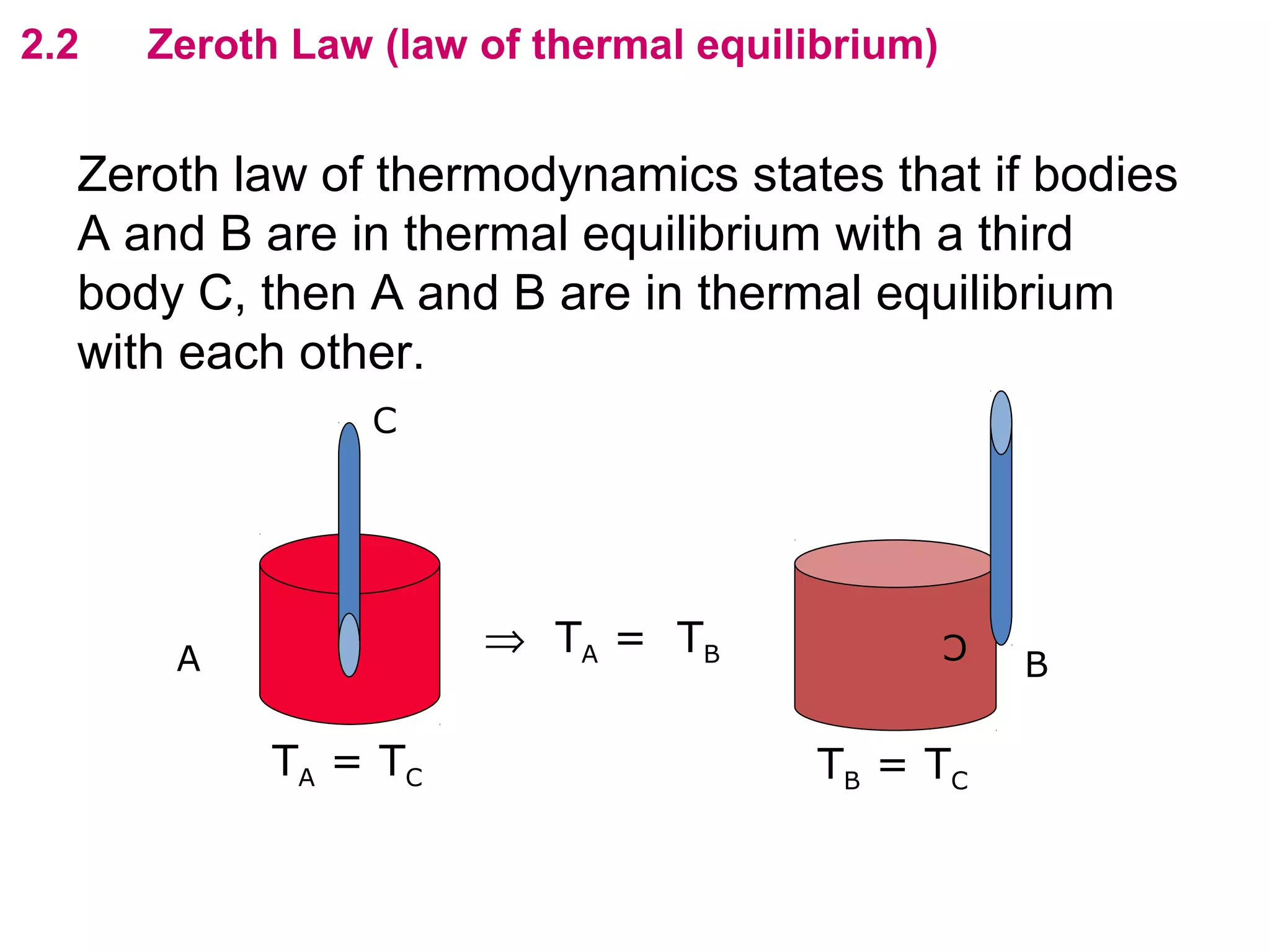
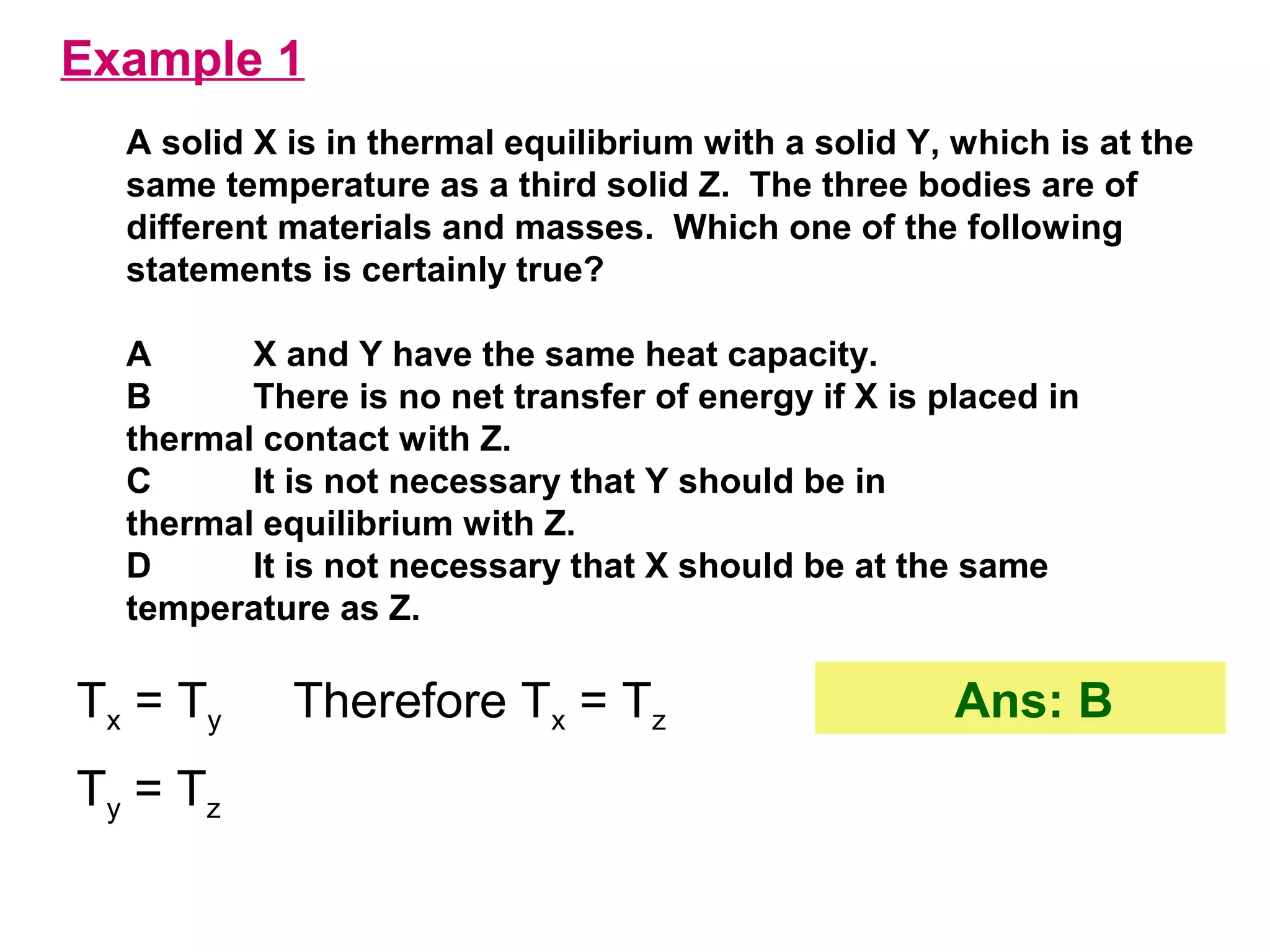
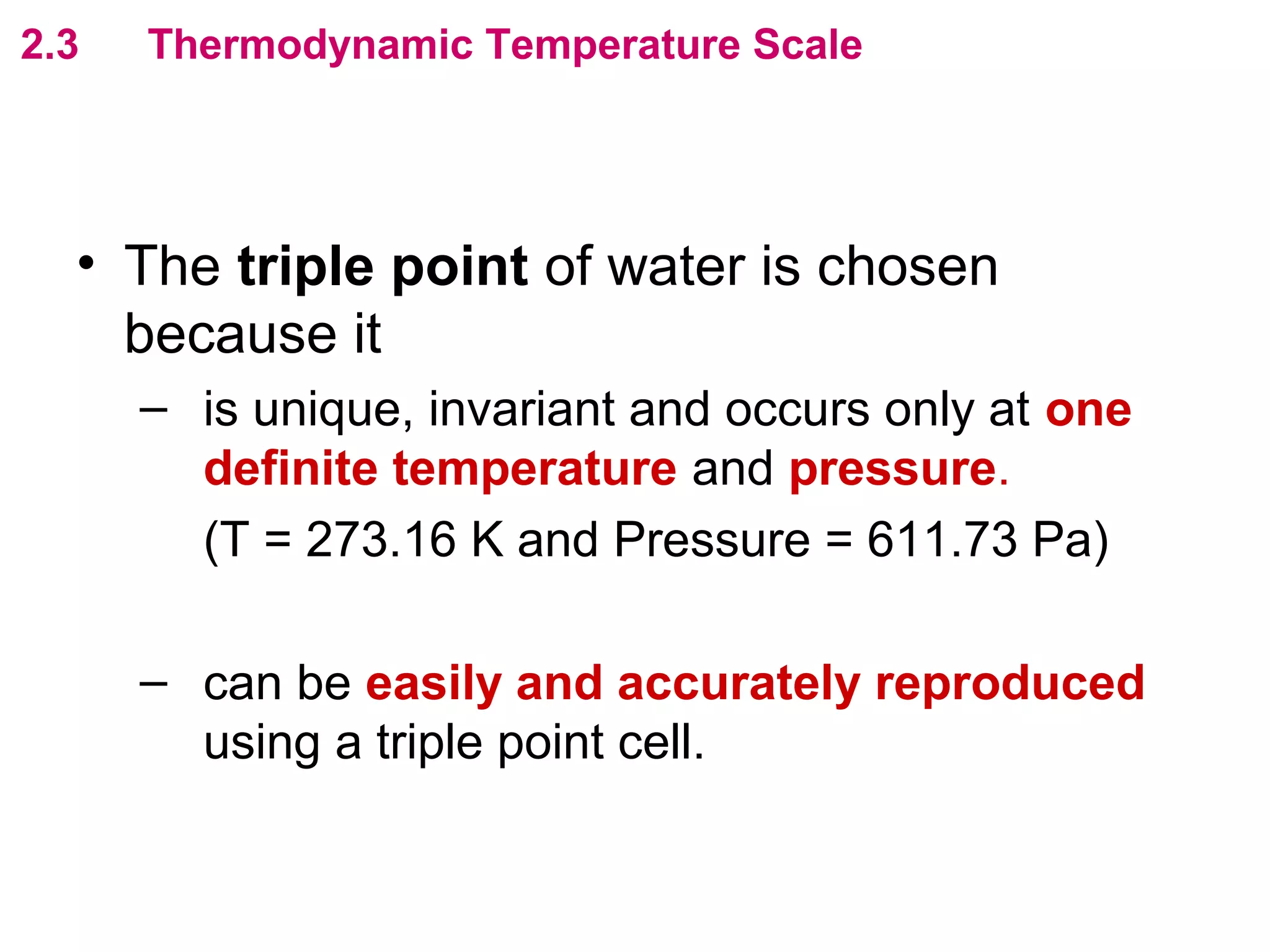
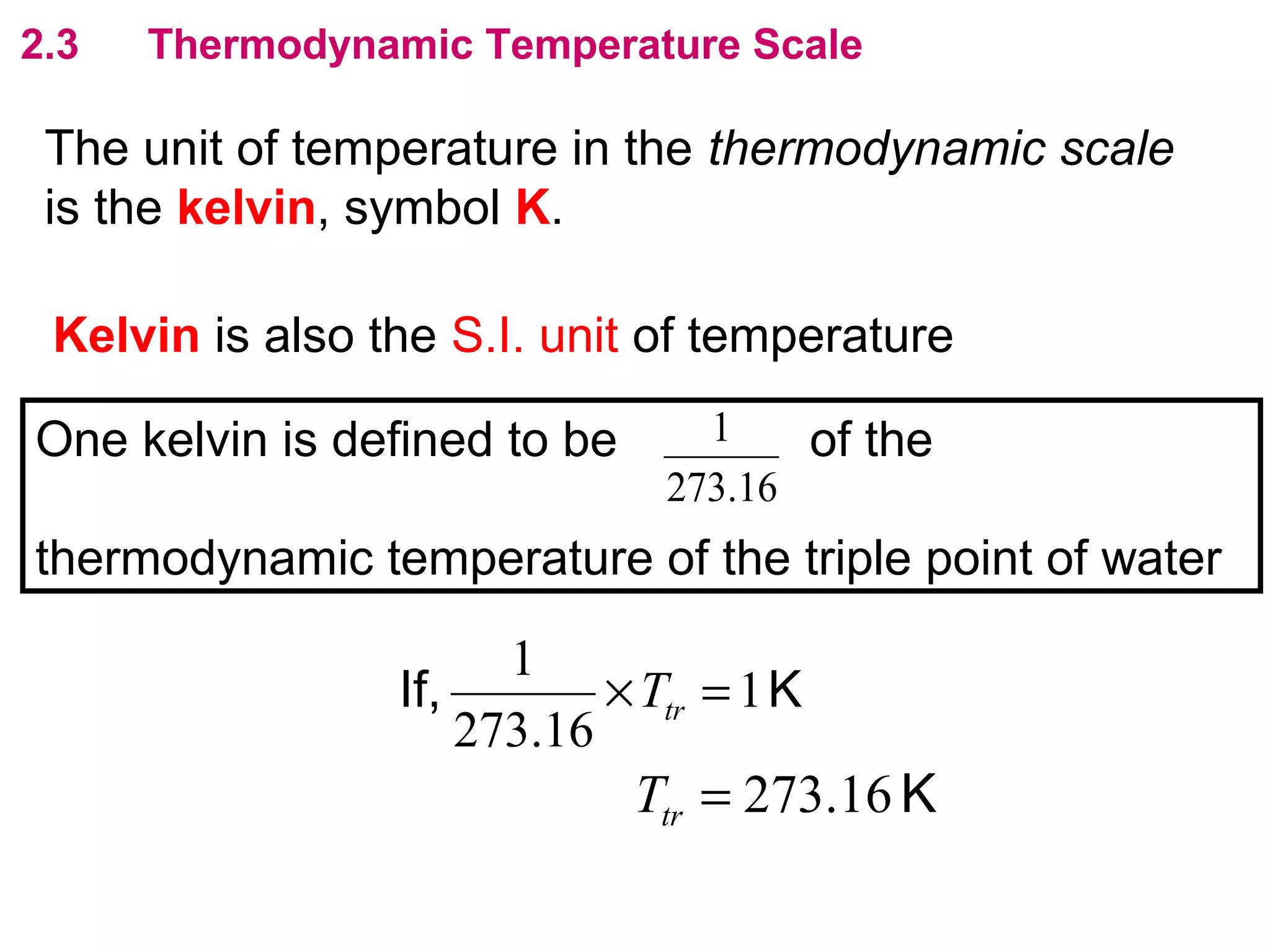
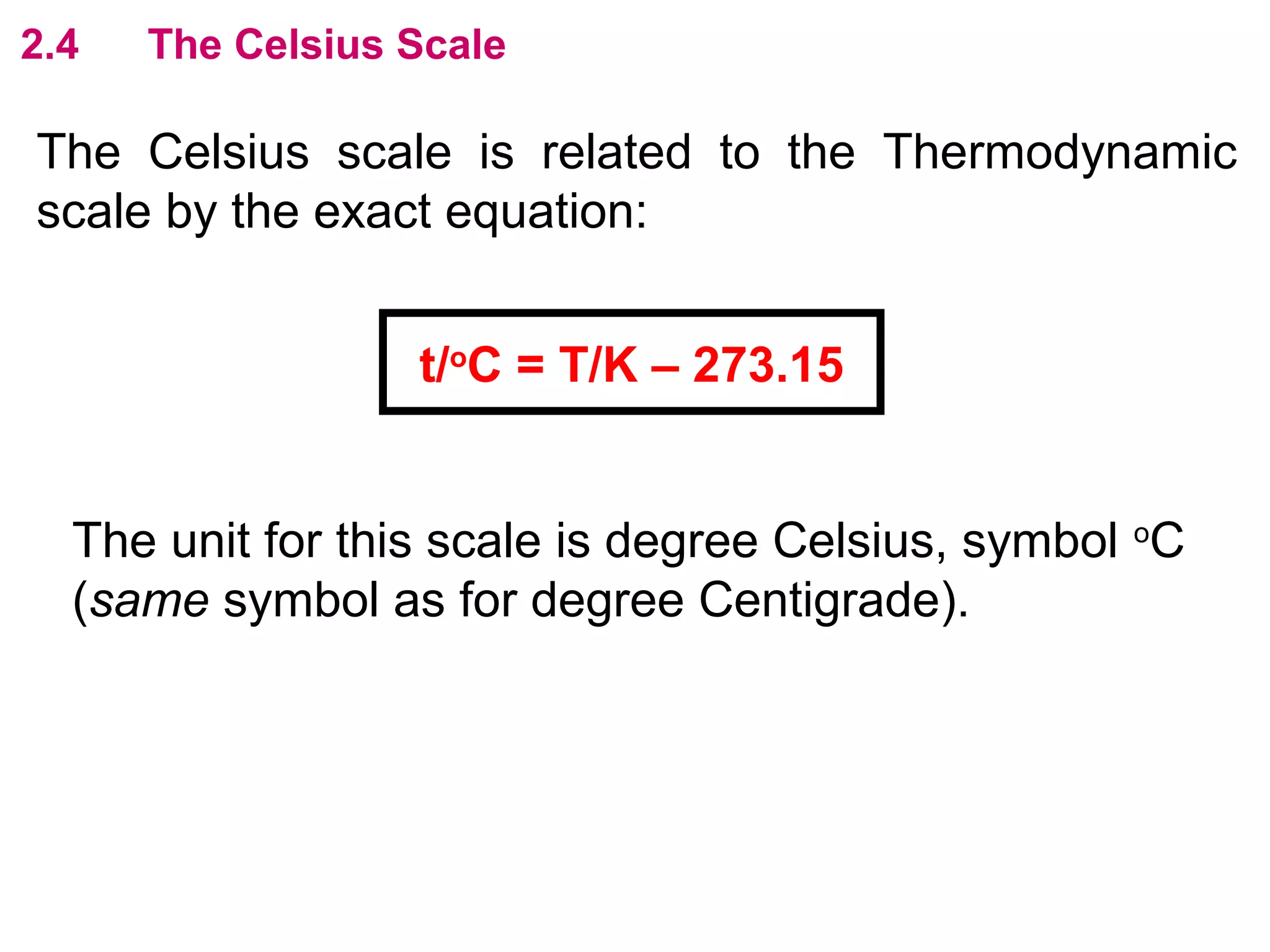
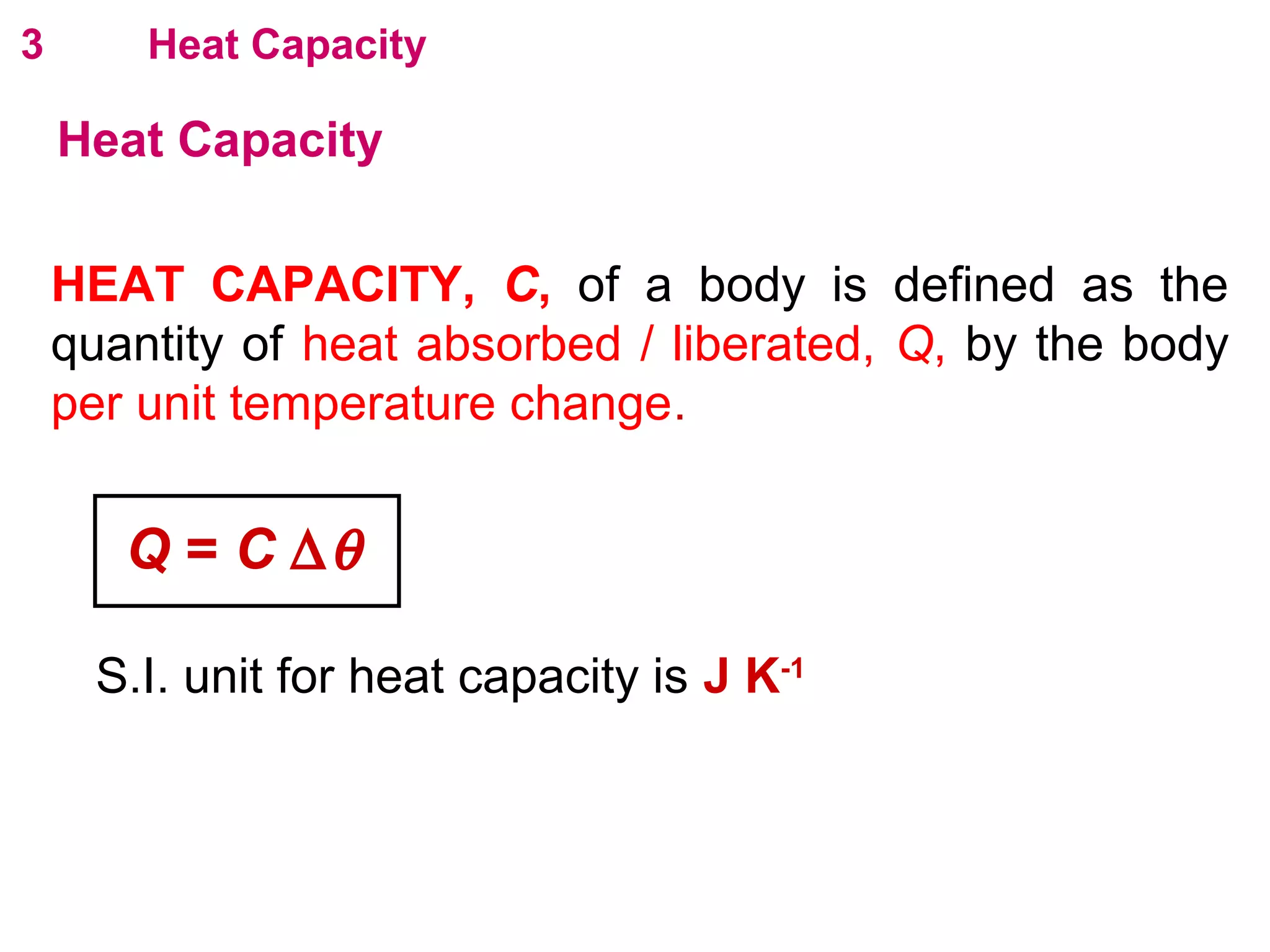
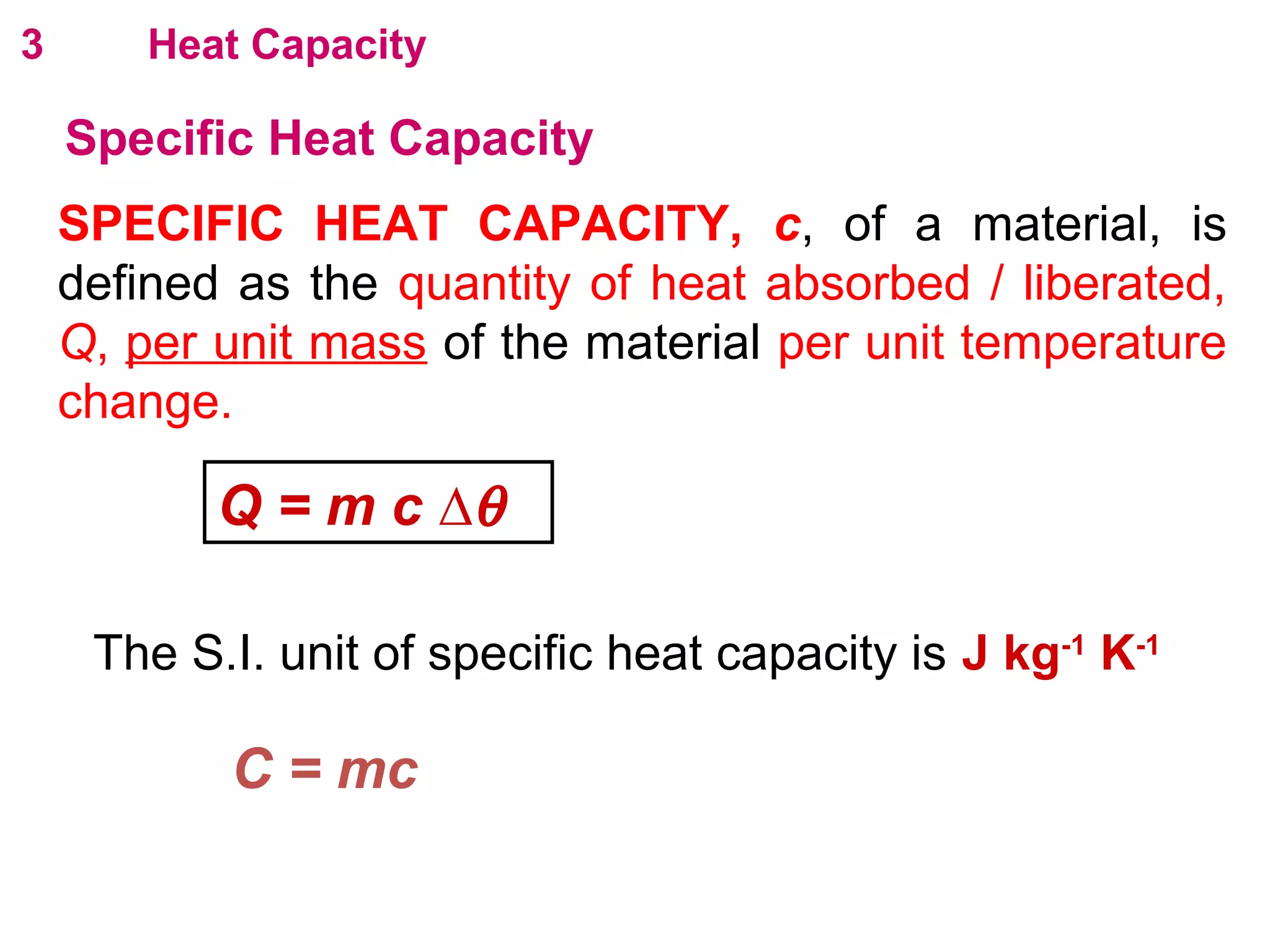
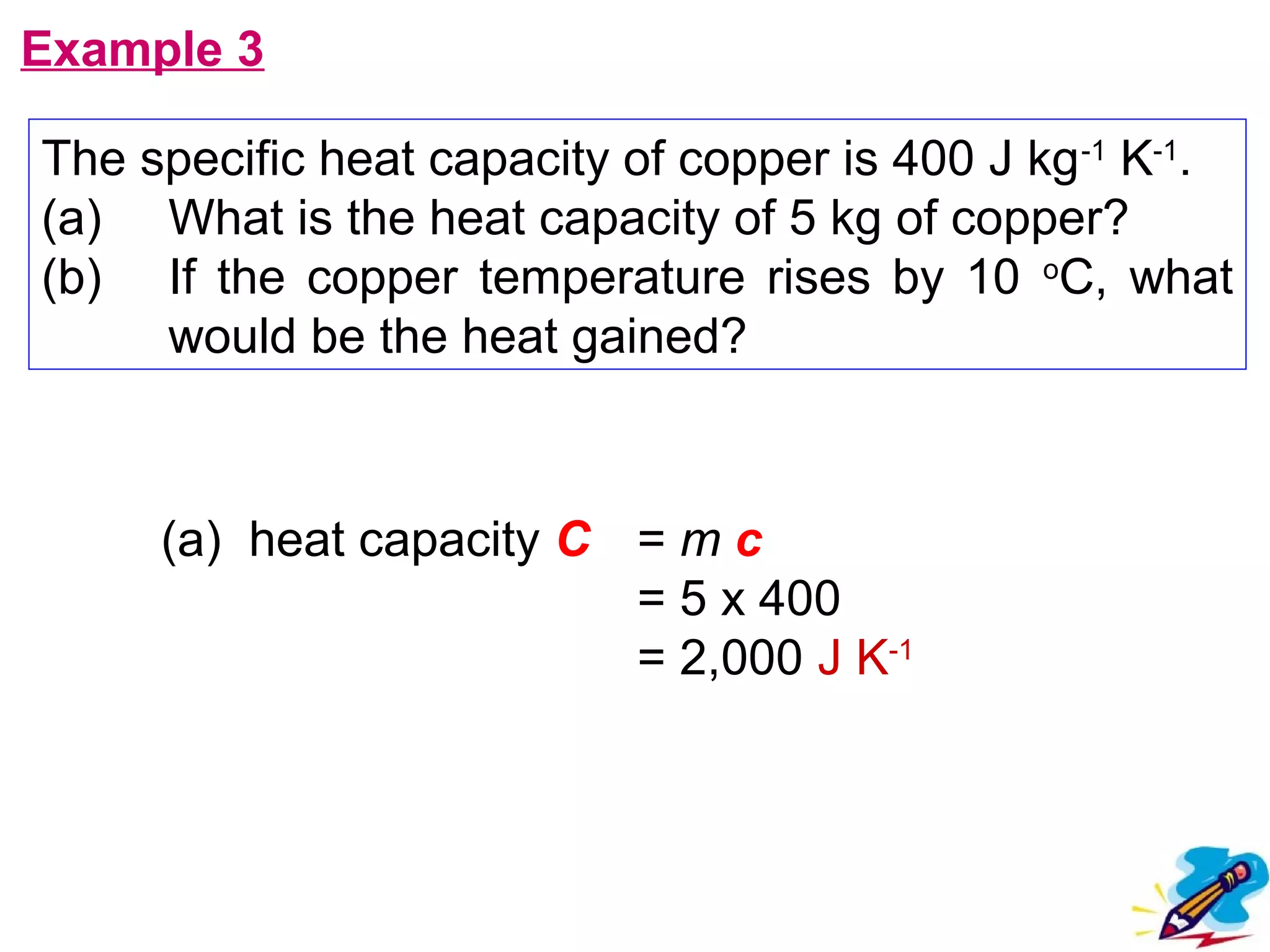
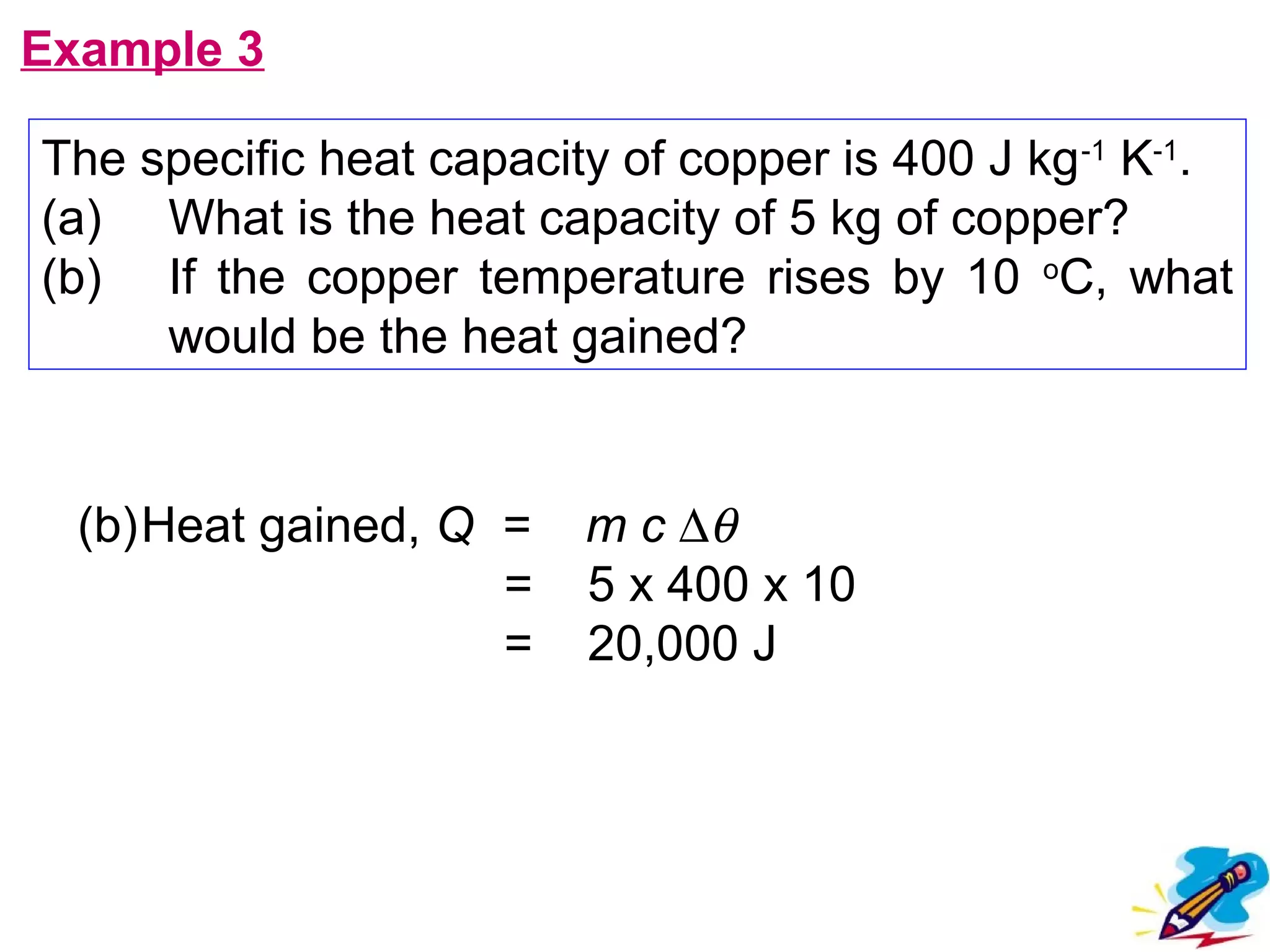
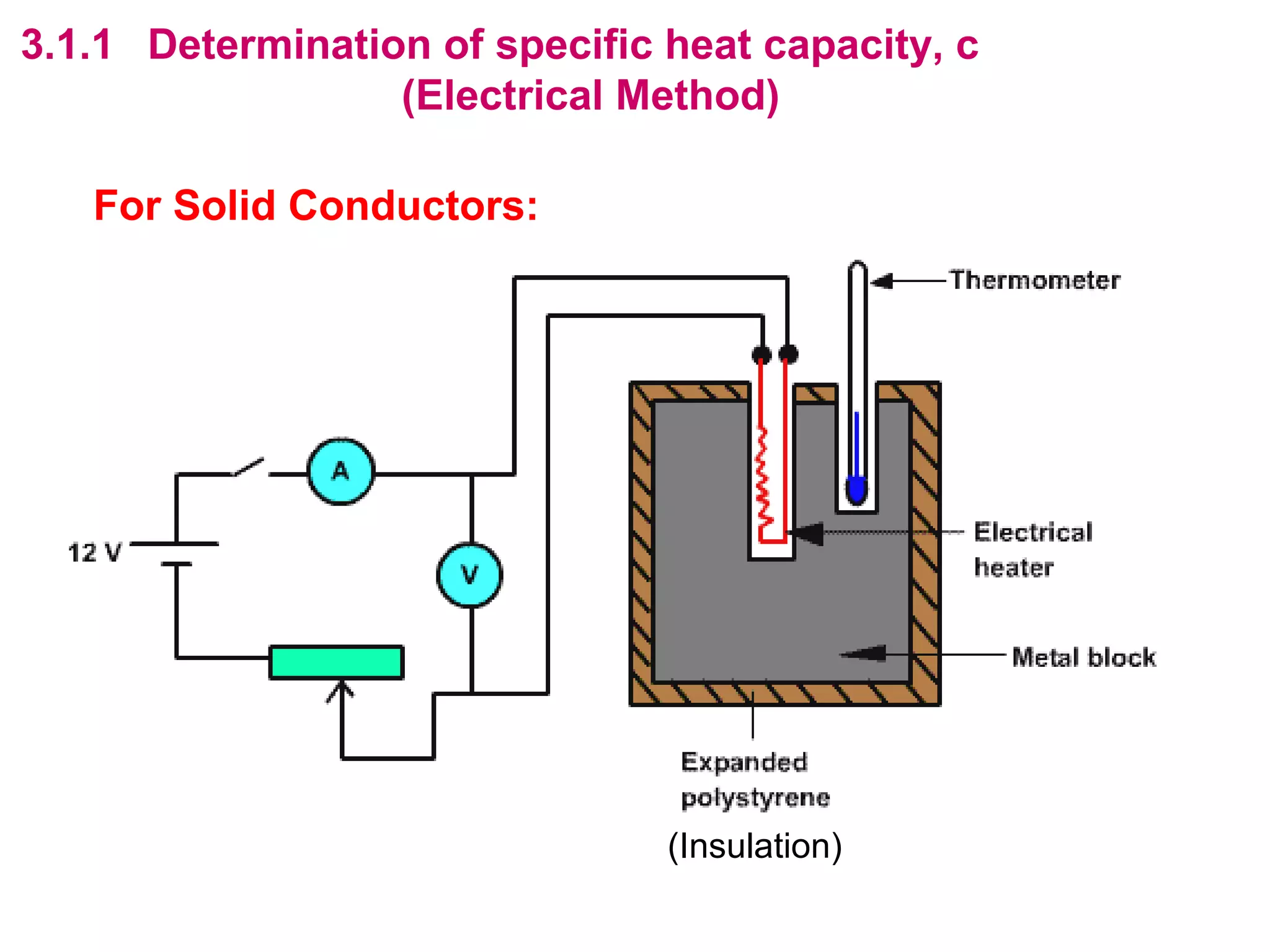
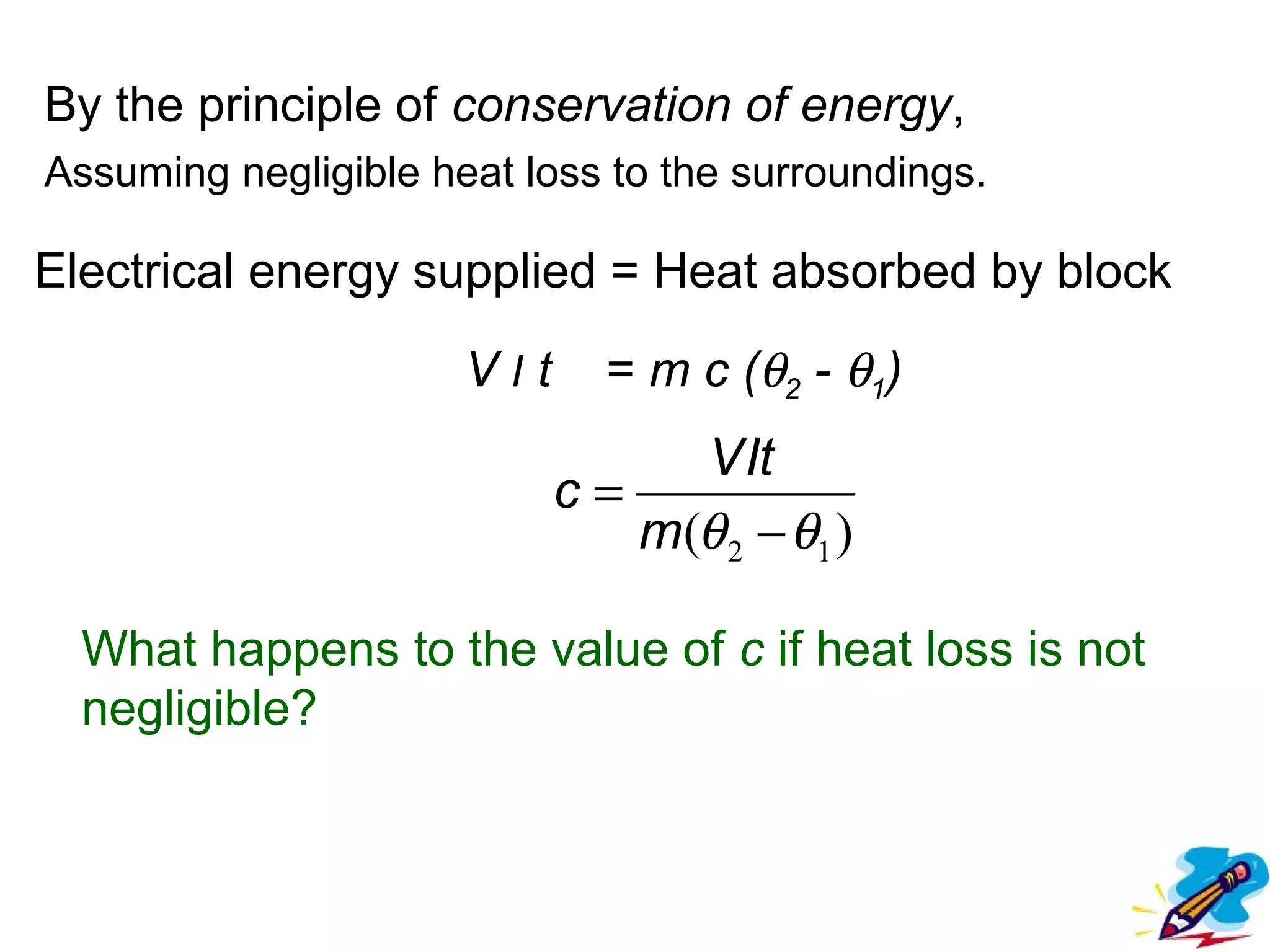
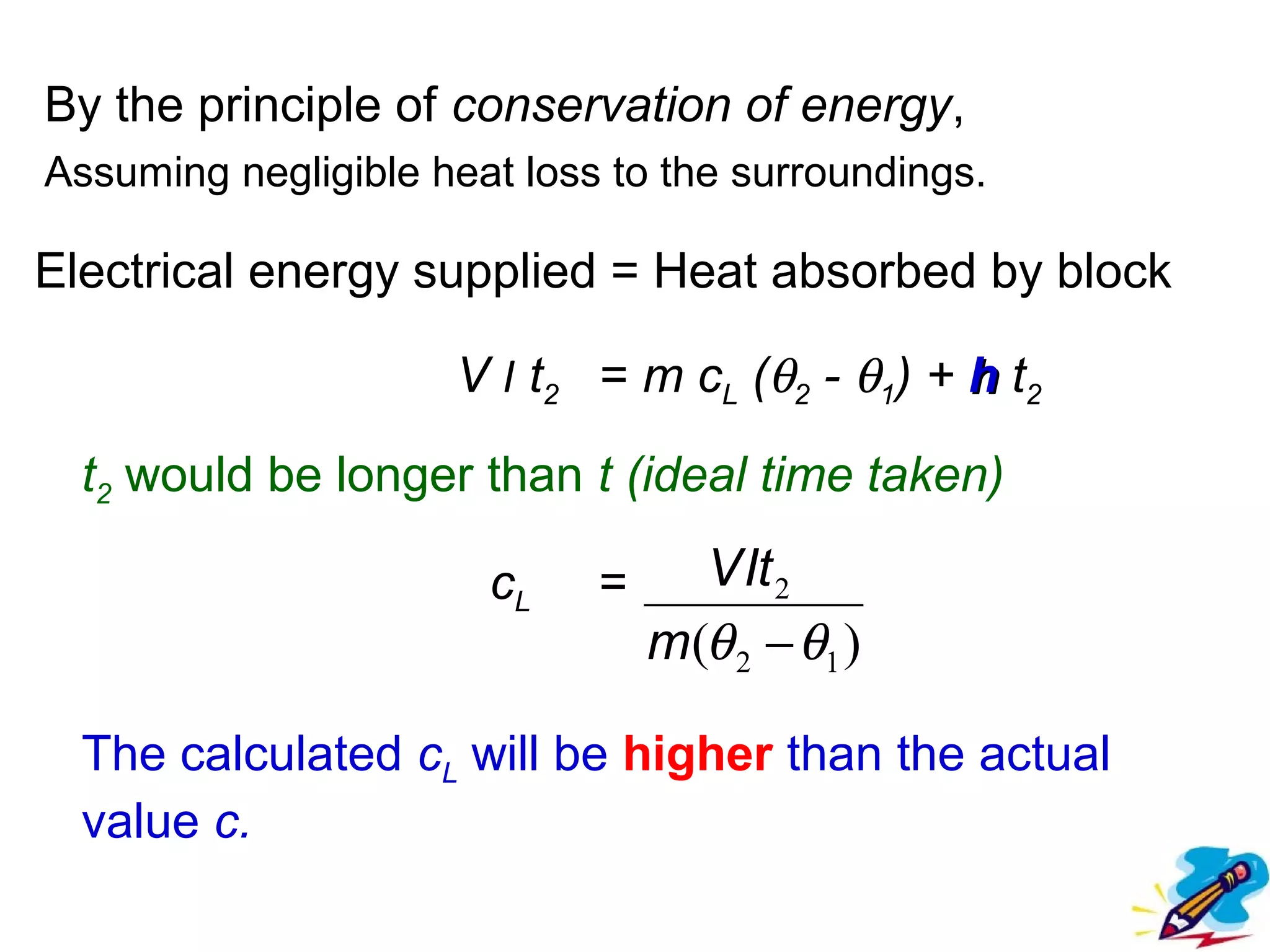
The calculated specific heat capacity cL would be higher than the actual specific heat capacity c if heat loss is not negligible. This is because some of the electrical energy supplied is lost to the surroundings through heat loss, rather than being fully absorbed by the block. So a longer time t2 is required to raise the temperature by the same amount, resulting in an overestimation of c.