The document discusses atomic structure and the development of atomic theory. It explains that:
1) All matter is made of tiny indivisible particles called atoms, as proposed by Democritus in ancient Greece.
2) Laws of conservation of mass and constant composition provided evidence supporting atomic theory by showing matter is neither created nor destroyed in chemical reactions.
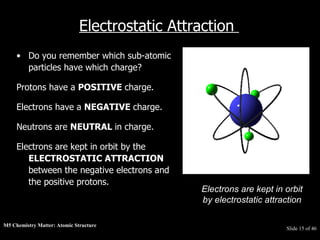
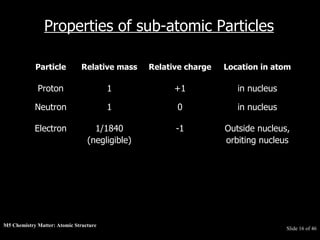
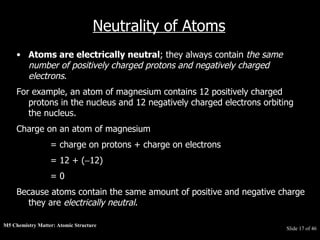
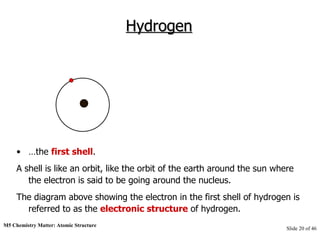
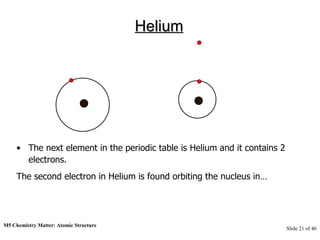
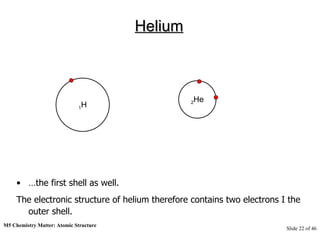
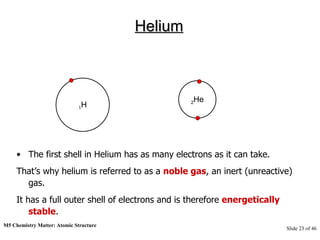
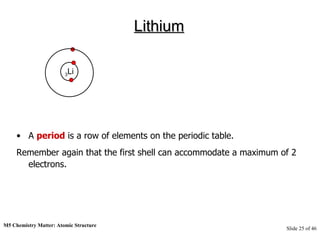
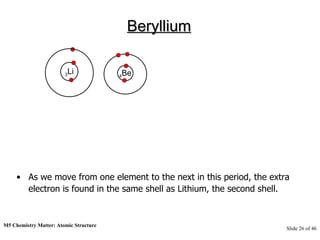
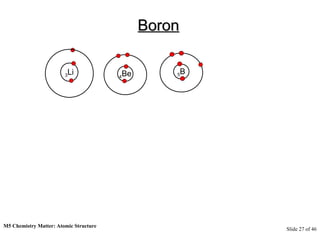
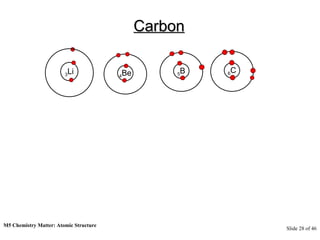
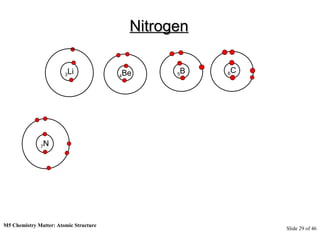
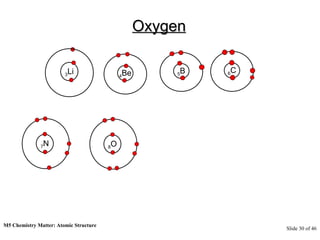
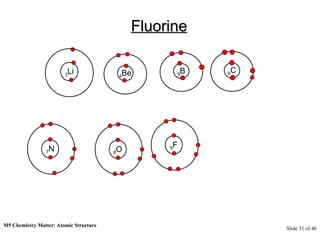
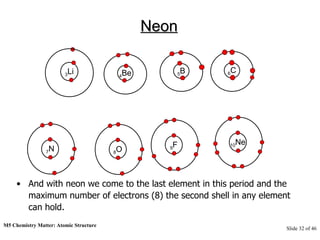
3) Atoms are composed of even smaller subatomic particles - electrons orbiting a dense nucleus of protons and neutrons, held together by electrostatic attraction. The identity of an element is determined by its number of protons.















![M5 Chemistry Matter: Atomic Structure Slide of 46 Practice Questions 1. J05/2/5f. Astatine, At, is below iodine in Group VII of the Periodic Table. (i) How many protons does astatine have in its nucleus? [1] (iii) The most common isotope of astatine has a nucleon number (mass number) of 210. Calculate the number of neutrons in this isotope of astatine. [1]](https://image.slidesharecdn.com/3mypatomicstructure-101014112415-phpapp01/85/3-myp-chemistry-atomic-structure-39-320.jpg)
![M5 Chemistry Matter: Atomic Structure Slide of 46 Practice Questions 2. J04/2/5. Look at the list of five elements below. argon bromine chlorine iodine potassium (a) Put these five elements in order of increasing proton number. [1] (b) Put these five elements in order of increasing relative atomic mass. [1] (c) The orders of proton number and relative atomic mass for these five elements are different. Which one of the following is the most likely explanation for this? Tick one box. The number of protons must always equal the number of neutrons. The atoms easily gain or lose electrons. The presence of neutrons. The proton number of a particular element may vary.](https://image.slidesharecdn.com/3mypatomicstructure-101014112415-phpapp01/85/3-myp-chemistry-atomic-structure-40-320.jpg)


![M5 Chemistry Matter: Atomic Structure Slide of 46 Practice Questions 5. Use a periodic table to help you answer this question. This question is about elements and atoms. (a) About how many different elements are found on Earth? Draw a ring around the correct number. 40 50 60 70 80 90 [1] (b) The following are parts of an atom: [3] electron neutron nucleus proton Choose from the list the one which: (i) has no electrical charge; (ii) contains two of the other particles; (iii) has very little (negligible) mass.](https://image.slidesharecdn.com/3mypatomicstructure-101014112415-phpapp01/85/3-myp-chemistry-atomic-structure-43-320.jpg)


