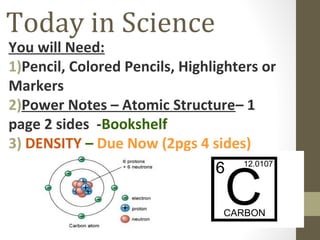
This document provides an overview of the history and development of atomic structure and the atomic model. It discusses early Greek philosophers like Democritus who proposed that all matter is made of invisible indivisible particles called atoms. It then summarizes key discoveries and models proposed by scientists like Dalton, Thomson, Rutherford, Bohr, Schrodinger that refined our understanding of atomic structure, such as discovering the electron, proposing the planetary model of electrons orbiting the nucleus, and the wave mechanical model. The document also defines the basic subatomic particles of protons, neutrons and electrons, isotopes, and provides examples of how atomic mass and ions are related to atomic structure.