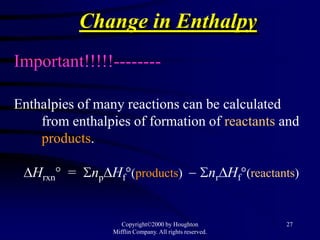
The document is from an honors chemistry textbook and discusses thermochemistry, specifically:
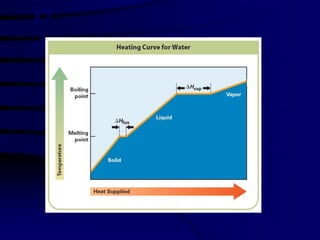
1) The molar heat of fusion and solidification, and how the heat absorbed during melting equals the heat released during solidification.
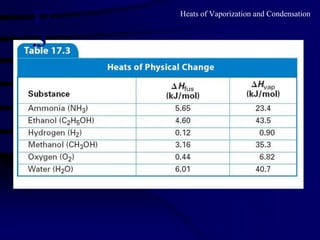
2) The molar heat of vaporization and condensation, and how the heat required for vaporization equals the heat released during condensation.
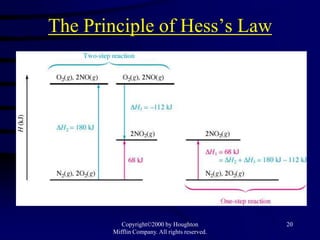
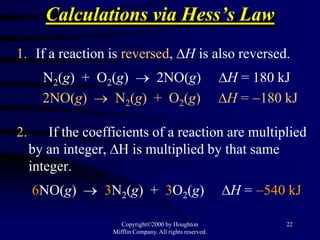
3) Hess's law, which states that the enthalpy change of a reaction is the same whether it occurs in one step or multiple steps.



















![Standard States
Compound
For a gas, pressure is exactly 1 atmosphere.
For a solution, concentration is exactly
1 molar.
Pure substance (liquid or solid), it is the pure
liquid or solid.
Element
The form [N2(g), K(s)] in which it exists at
1 atm and 25°C.
Copyright©2000 by Houghton 26
Mifflin Company. All rights reserved.](https://image.slidesharecdn.com/chapter17thermochemistrysections17-317-4-120428132956-phpapp01/85/Chapter-17-thermochemistry-sections-17-3-17-4-26-320.jpg)