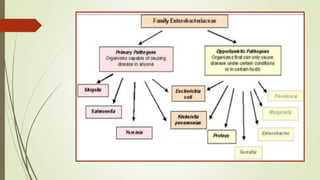
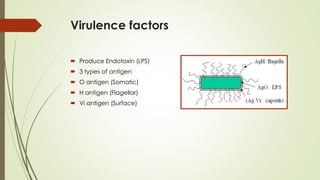
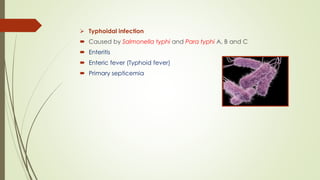
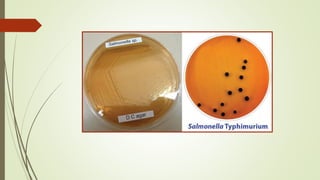
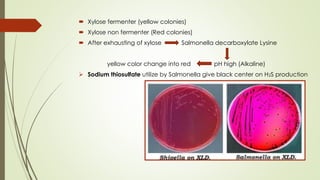
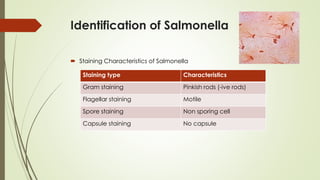
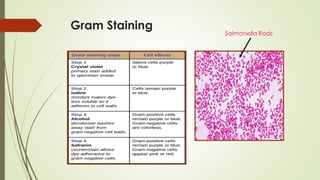
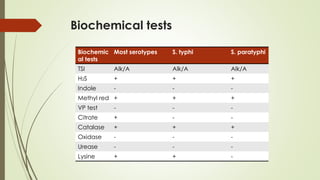
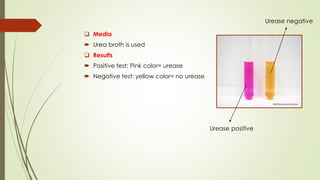
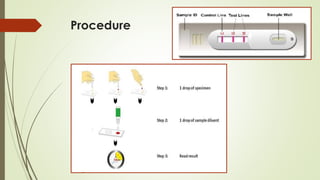
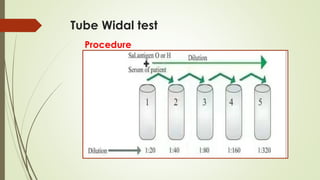
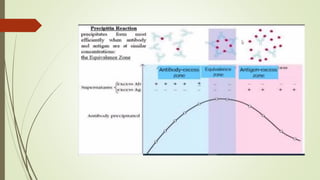
The document discusses the isolation and identification of Salmonella species, particularly focusing on their classification, historical aspect, virulence factors, and methods for sampling and identification. It highlights the importance of Salmonella enterica in causing human infections, both non-typhoidal and typhoidal, as well as the various serotypes and their effects on animals. Additionally, it covers diagnostic tests including biochemical and serological methods to detect Salmonella and outlines the implications of multi-drug resistance in food sources.