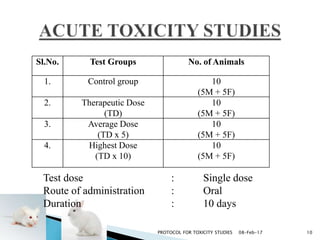
This document outlines protocols for toxicity studies of herbal medicines. It discusses types of toxicity studies including acute, sub-acute, chronic and subchronic studies. It describes commonly used laboratory animals for such studies like mice, rats, rabbits and protocols for acute, subacute and chronic toxicity studies. The document provides an example of a toxicity study report format and discusses guidelines for assessing toxicity of herbal medicines from WHO.