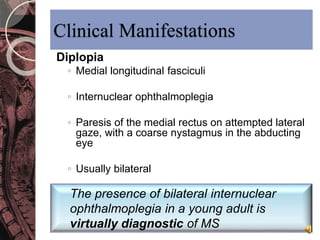
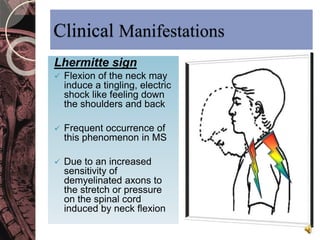
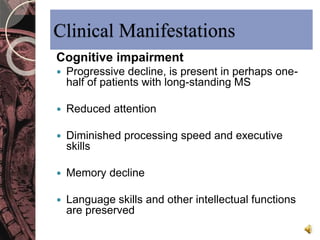
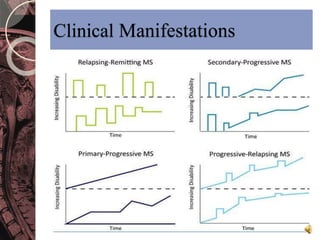
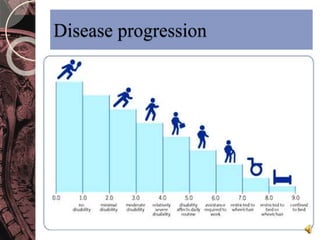
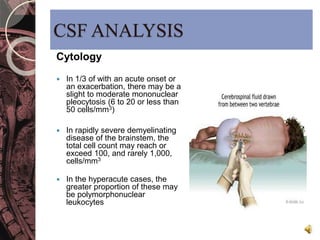
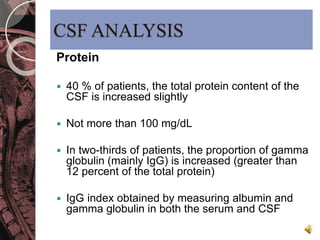
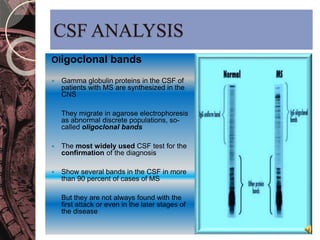
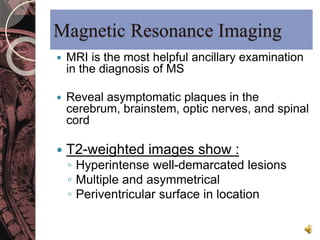
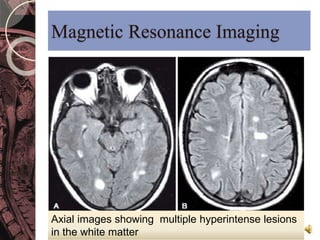
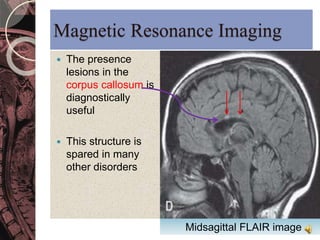
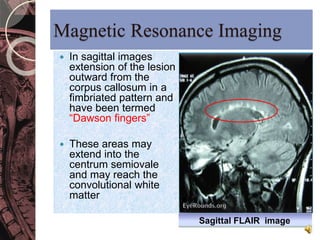
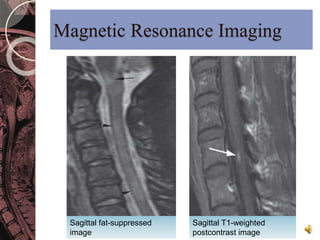
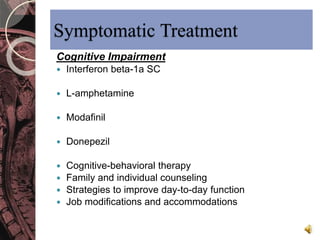
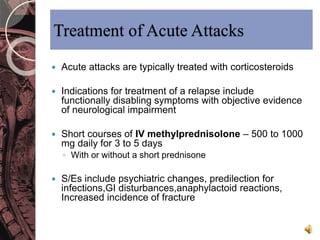
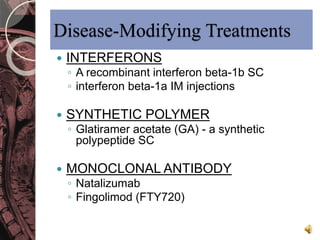
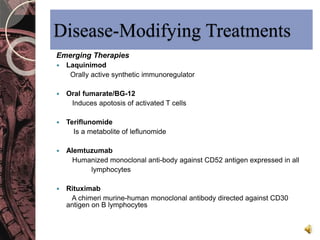
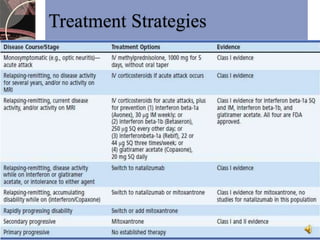
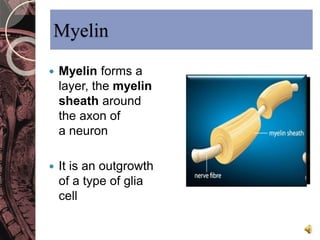
This document discusses myelin, its composition and function. It describes how myelin forms a sheath around neurons, insulating them and allowing rapid nerve impulse propagation. Cholesterol and proteins like myelin basic protein are important myelin components. Myelination begins in fetal development and continues through life. Diseases like multiple sclerosis involve demyelination through autoimmune or other processes. The document examines MS in terms of epidemiology, pathogenesis, clinical presentation and investigations like CSF analysis and MRI that are used in diagnosis.





![Pathogenesis
Infection
Little direct evidence supports the concept of a
role for viral infection
Human T-cell lymphotropic virus type 1 [HTLV1]
Human herpesvirus 6 (HHV6)
Epstein-Barr virus (EBV)
Chlamydia pneumoniae
Environmental Factors
Sunlight exposure during growth
Vitamin D
Epidemiological data supportive](https://image.slidesharecdn.com/1-141102051233-conversion-gate01/85/1-multiple-sclerosis-22-320.jpg)