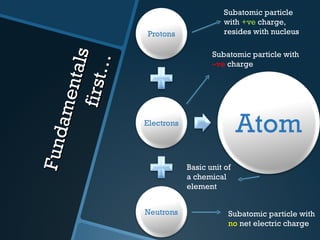
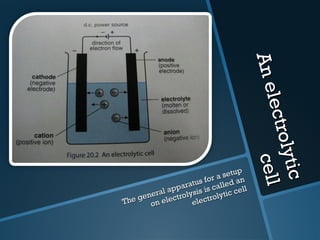
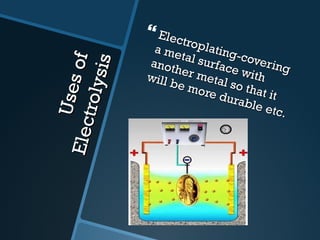
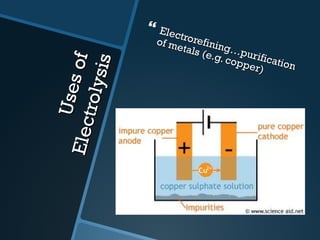
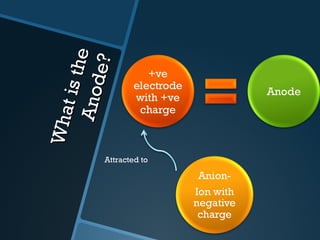
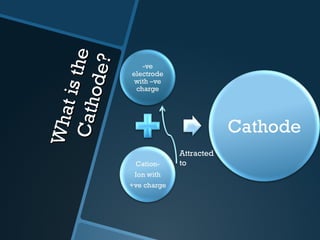
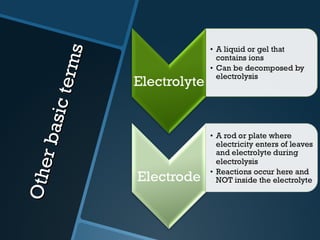
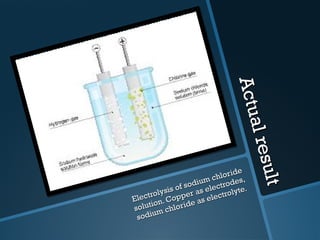
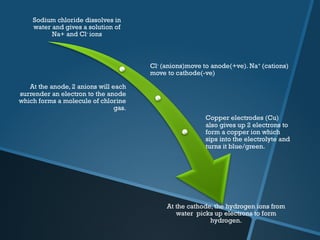
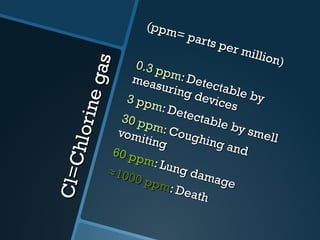
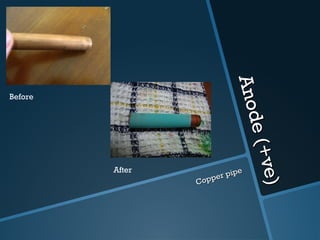
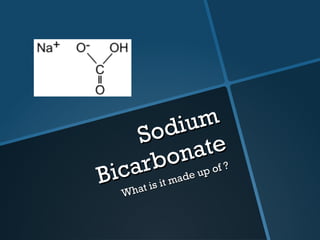
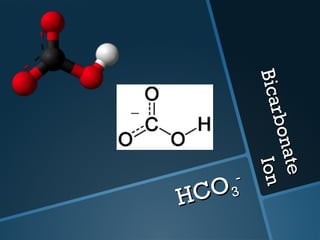
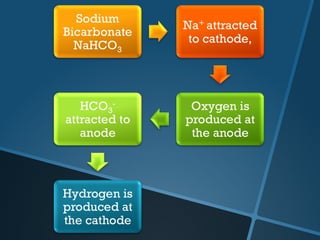
The document discusses electrolysis, including its uses, basics, history, experiments conducted, and potential dangers. It describes two electrolysis experiments conducted at home - electrolysis of sodium chloride solution using copper electrodes, and electrolysis of sodium bicarbonate solution. Both experiments resulted in oxidation at the anode and reduction at the cathode. Potentially dangerous gases like chlorine and hydrogen were produced.