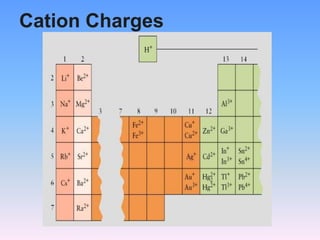
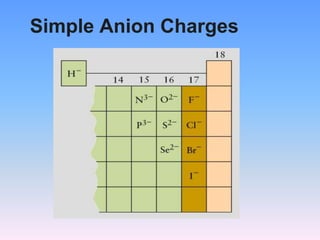
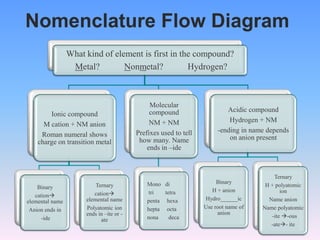
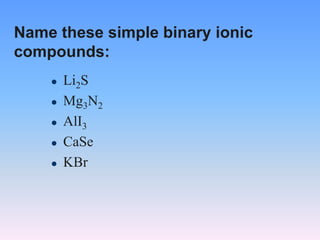
The document serves as a guide for naming and writing formulas for ionic compounds, covering key concepts such as cation charges, the use of Roman numerals for transition metals, and balancing charges. It includes examples and procedures for naming simple binary ionic compounds and writing their formulas, like lithium sulfide and magnesium nitride. Additionally, it features a riddle related to atoms and their electrons.