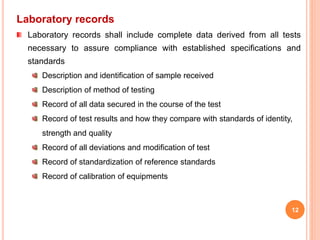
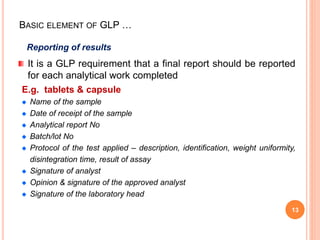
Good Laboratory Practice (GLP) guidelines provide standards for laboratory experiments and tests performed to support research, nonclinical studies, and regulatory submissions. The key goals of GLP are to ensure quality, reliability, and integrity of data through adherence to standard operating procedures, trained personnel, appropriate facilities and equipment, records management, and quality control. GLP aims to promote valid and robust research that can be reproduced internationally and supports regulatory review and decision making.






















![ASSAY EXAMPLES:
1. Assay of paracetamol tablets was done using HPLC and the following results
were obtained
Data
Weight of 20 tablets=12.2243 g
Weight of tablet powder taken for assay=152.5 mg
Stated content per tablet=500 mg
Original volume=200 ml
Dilution steps
• 20 ml into 100 ml
• 10 ml into 100 ml
• Calibration curve for paracetamol: Y= 35656X + 80 where X is in mg/100 ml
• Area of peak obtained for paracetamol in diluted sample extract =44 519
I. Calculate the percentage of stated content in paracetamol tablets
II. Does the product pass the QC test for assay? [BP limit: 95%-105%]](https://image.slidesharecdn.com/02goodlaboratorypracticeglp1-220912174713-dfb4540c/85/02Good-Laboratory-Practice-GLP-1-pptx-25-320.jpg)
![2. Assay of methyl dopa tablets was done as follow [BP, 2007].
10 tablets of methyl dopa were weighed and powdered. The quantity of powder
equivalent to 0.25 gm of methyl dopa was taken and dissolved in 0.05 M H2SO4 to
produce 100 ml and filtered using filter paper. 5 ml of the filtrate was taken and 2
ml of iron (II) sulphate –citrate solution and sufficient water was added to produce
100 ml. The absorbance of the resulting solution was measured at 550 nm and
the following results were obtained.
Stated content of methyl dopa per tablet=250 mg
Weight of 10 tablets=4.5 g
Absorbance reading of sample solution at 550 nm =0.425
Absorbance reading of standard solution(12.5 mg/100 ml) at 550 nm =0.445
a. Calculate the percentage of the stated amount of methyl dopa in the tablets
b.Does the product pass the Qc test for assay? [BP limit: 95%-105%]](https://image.slidesharecdn.com/02goodlaboratorypracticeglp1-220912174713-dfb4540c/85/02Good-Laboratory-Practice-GLP-1-pptx-26-320.jpg)