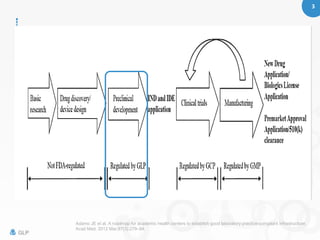
Good Laboratory Practice (GLP) is a quality system for managing non-clinical health and environmental safety studies, ensuring compliance with FDA and OECD standards to prevent fraud and protect public health. The document outlines the principles and requirements for GLP, including proper maintenance, calibration of equipment, and handling of chemicals and reagents in laboratory settings. It also highlights the importance of documentation and record-keeping for compliance and safe laboratory operations.