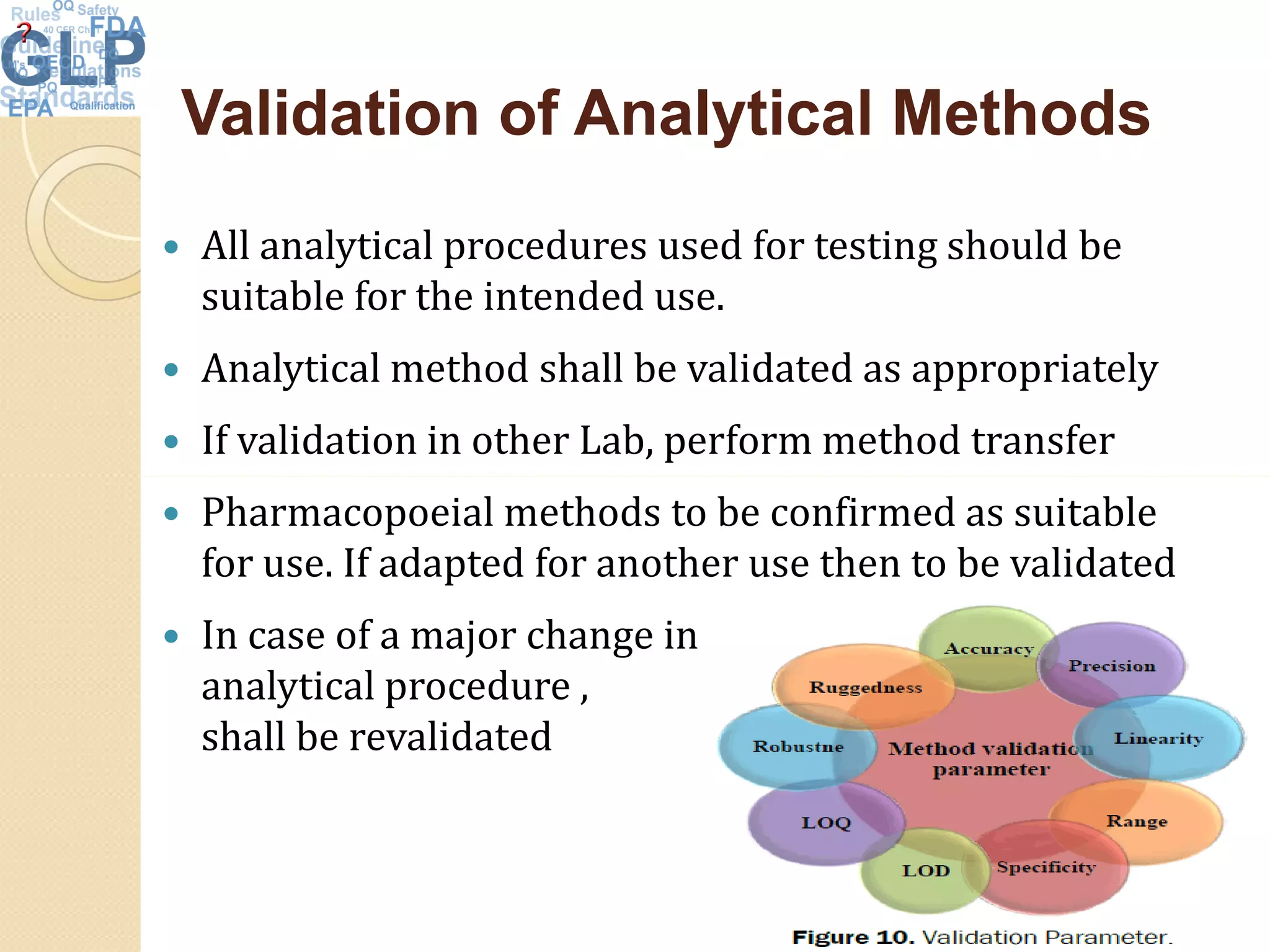
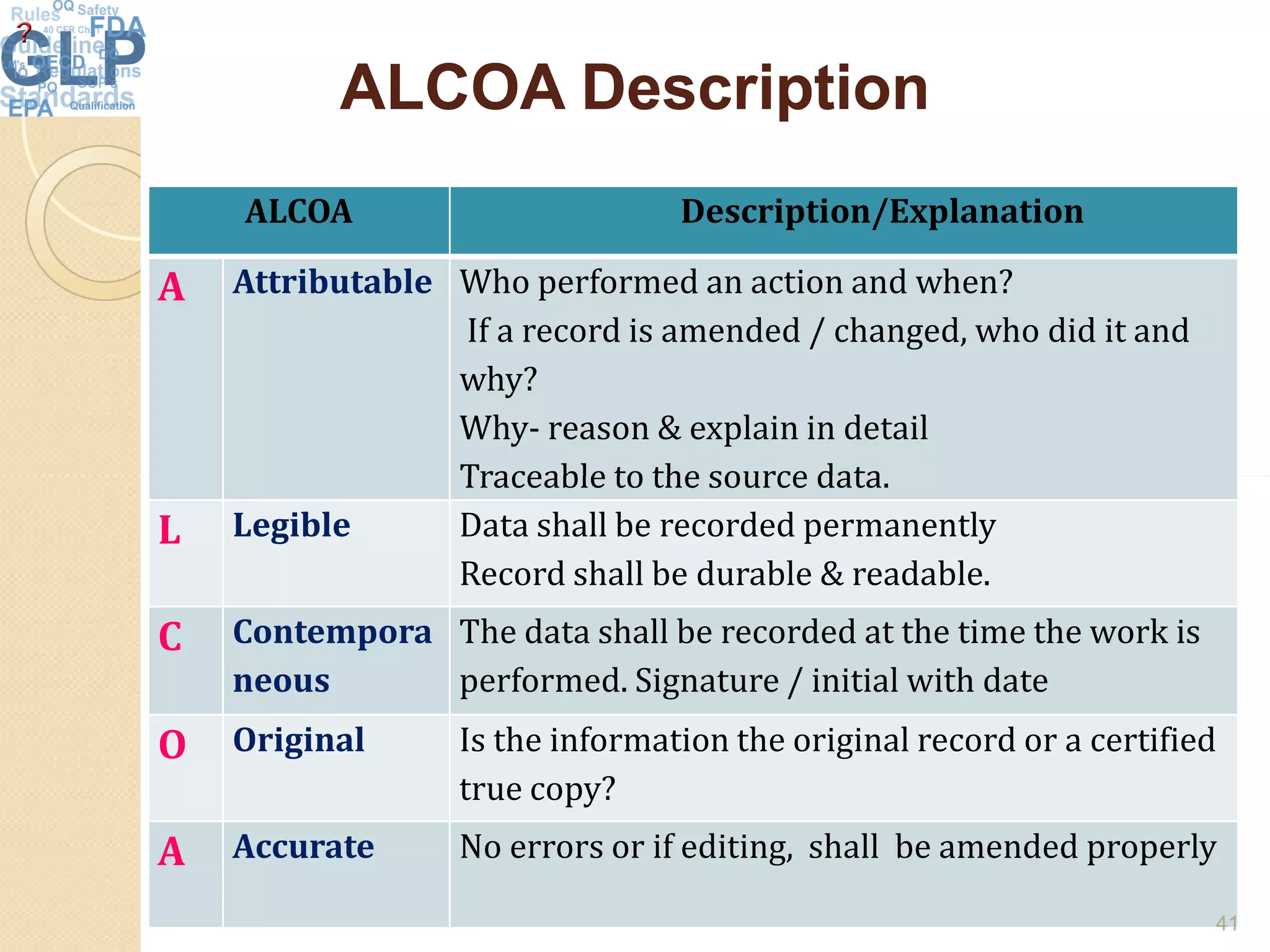
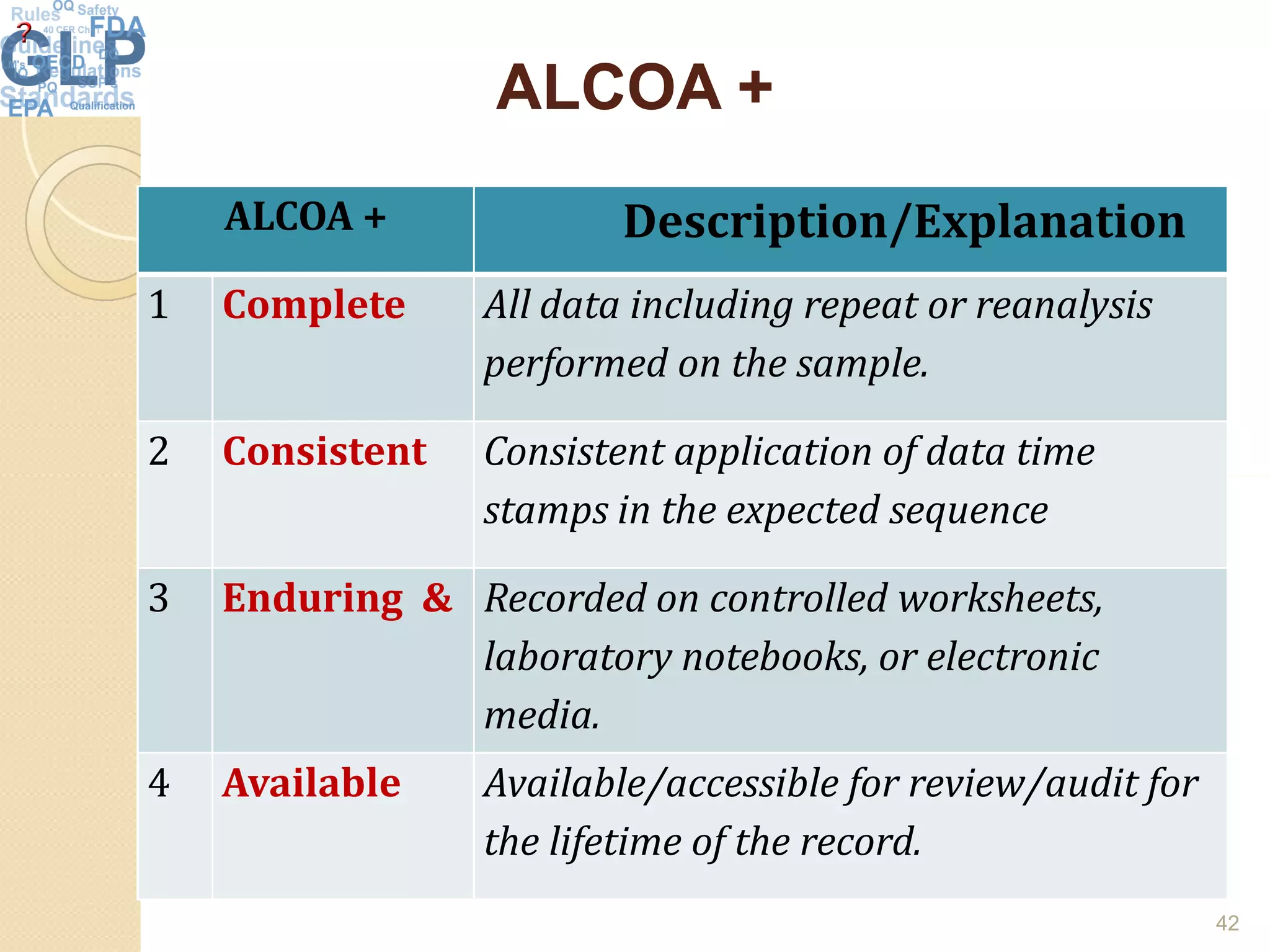
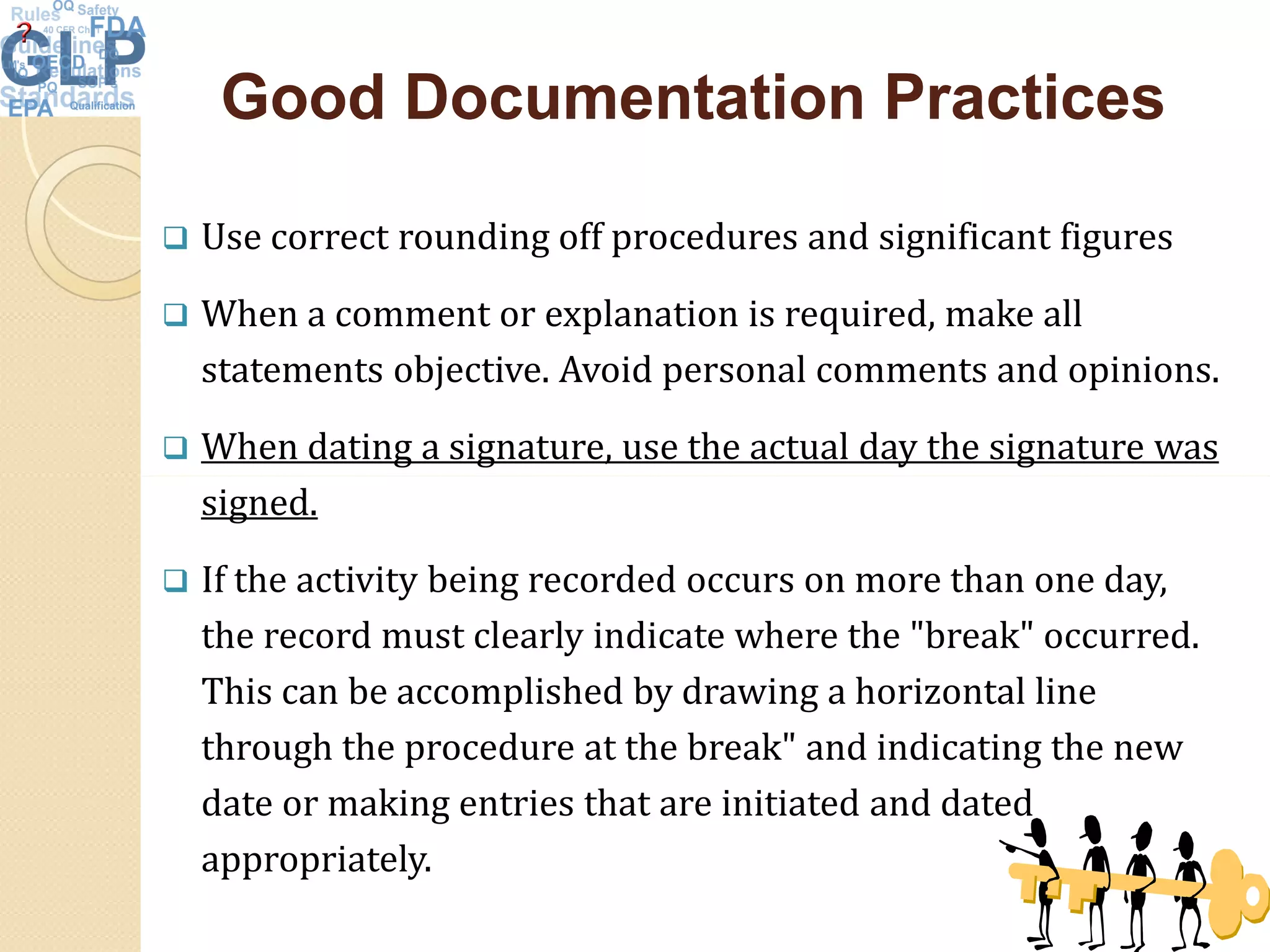
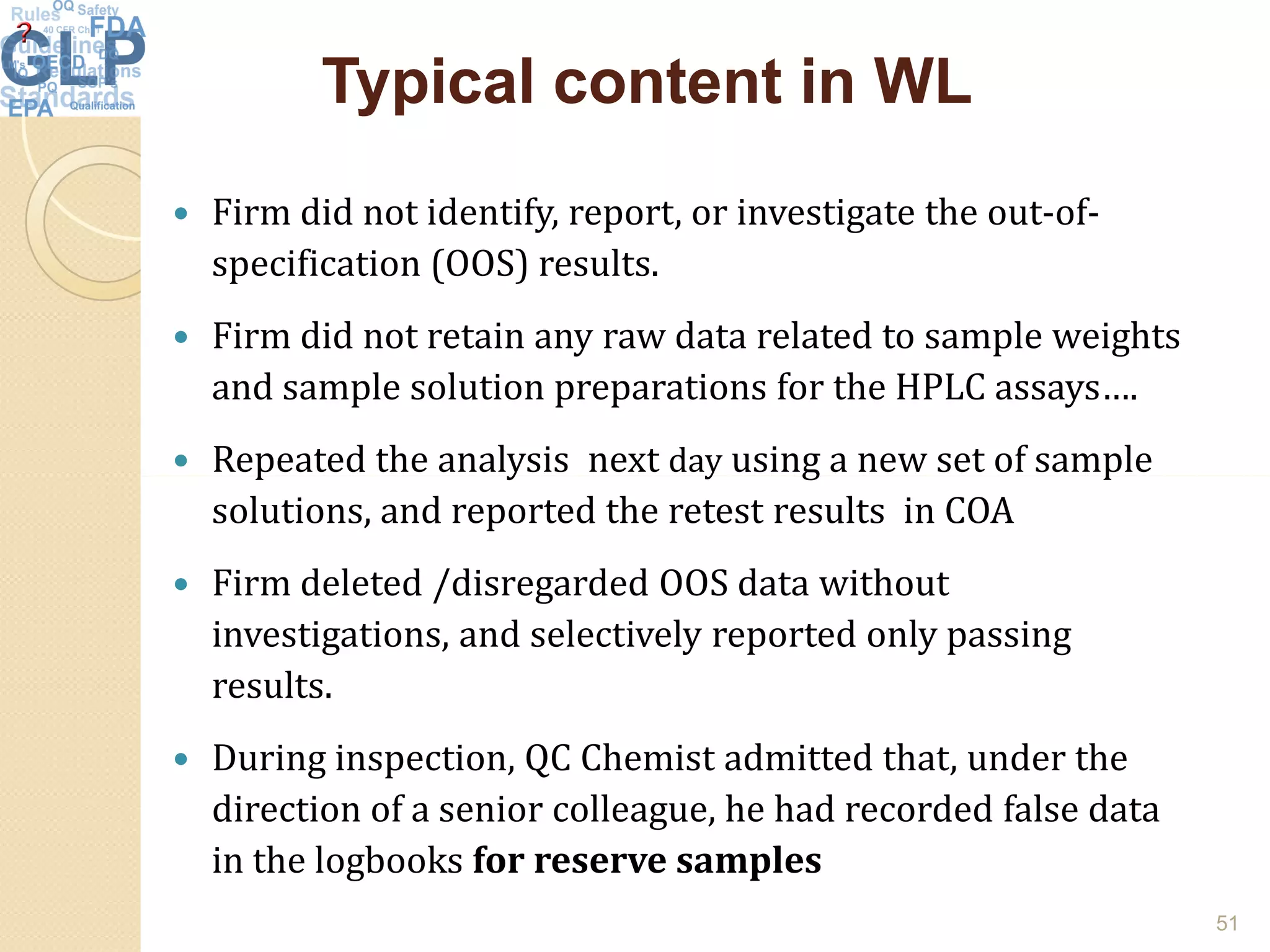
The document outlines good laboratory practices (GLP) in pharmaceutical quality control, emphasizing compliance with regulatory guidelines such as ICH Q7 and 21 CFR 210/211. Key areas covered include laboratory management, personnel qualifications, documentation and record-keeping, sample management, reagents, instruments, and validation processes. The document serves as a comprehensive framework for ensuring quality control and integrity in laboratory operations.