Embed presentation
Downloaded 1,121 times





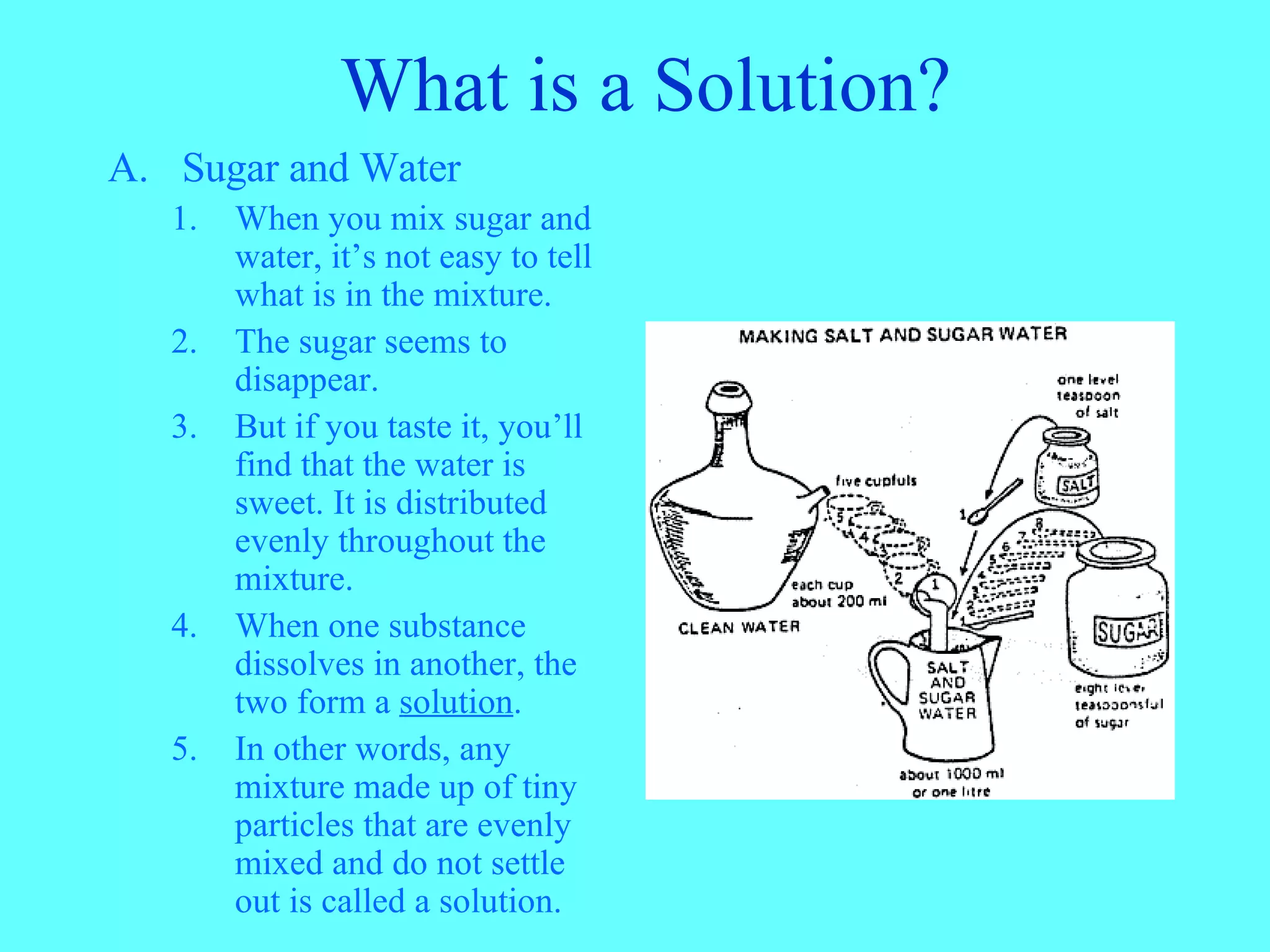

A mixture is a combination of two or more substances that retain their individual chemical and physical properties. There are two types of mixtures: homogeneous mixtures, where the substances are evenly mixed on a small scale and cannot be seen, and heterogeneous mixtures, where the individual substances can be visually identified. A solution is a type of homogeneous mixture where one substance dissolves evenly into another, such as sugar dissolving in water, forming a mixture on a molecular scale. Not all mixtures are solutions, as solubility determines whether a substance will dissolve in another.
Overview of lesson focusing on classifying mixtures and solutions.
A mixture is a combination of different kinds of matter keeping their properties. Examples include sugar and water.
Homogenous mixtures are mixed evenly, such as powdered drink mixes that do not separate.
Heterogenous mixtures are unevenly mixed, allowing for visibility of different substances, e.g., bread.
A solution forms when one substance dissolves in another, like sugar in water, creating a uniform mixture.
Clarification that while all solutions are mixtures, not all mixtures qualify as solutions; solubility defined.






