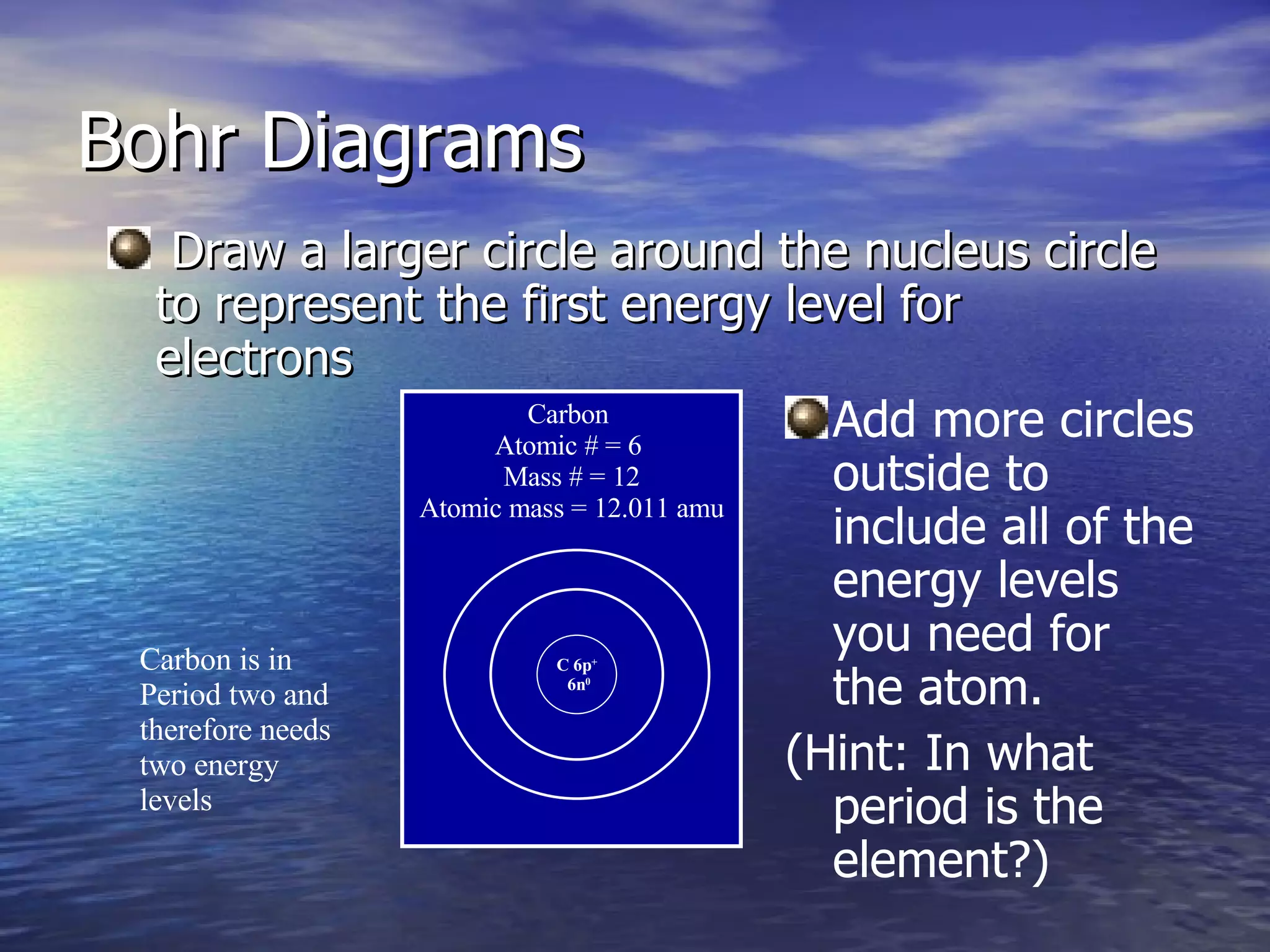
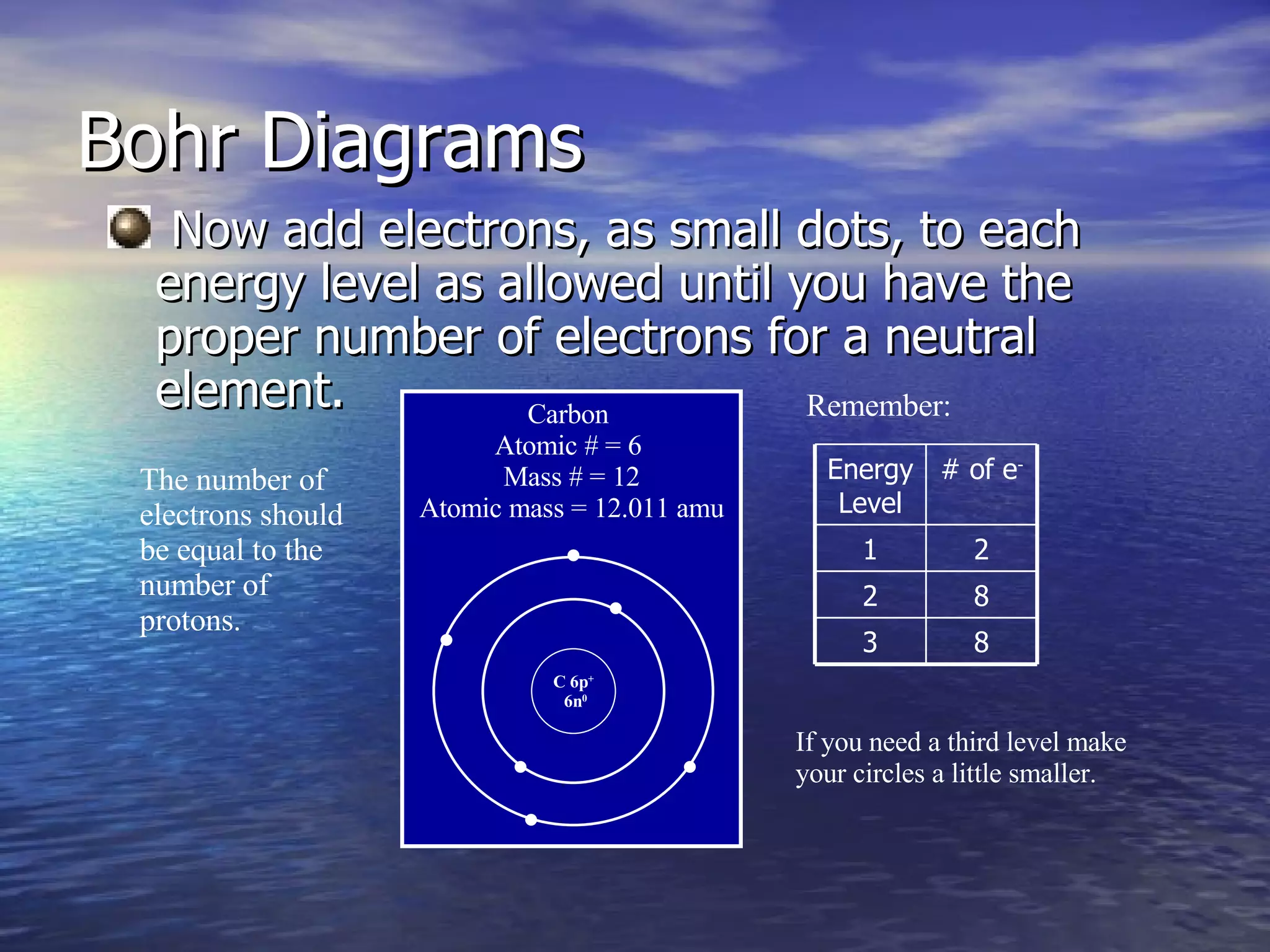
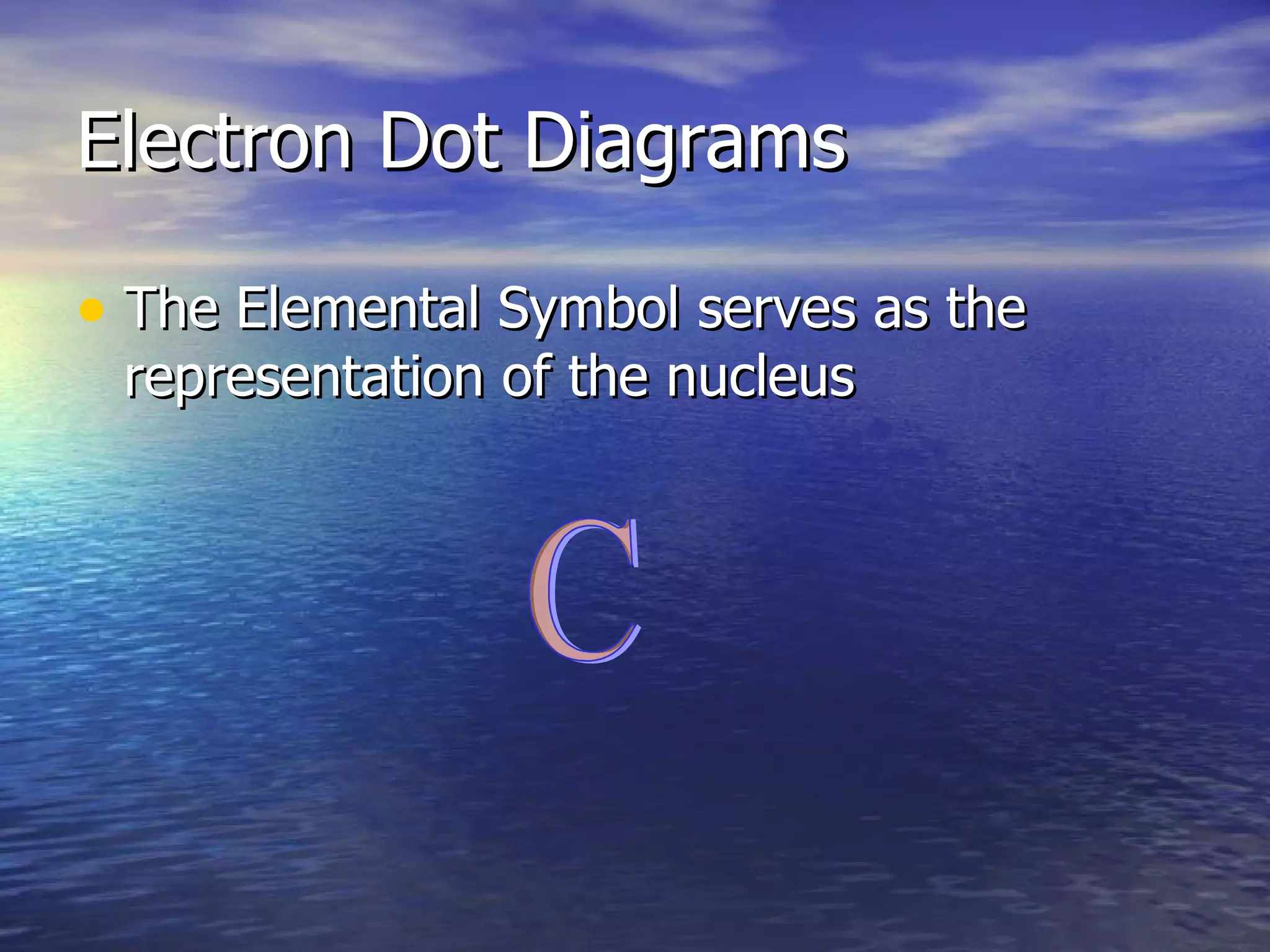
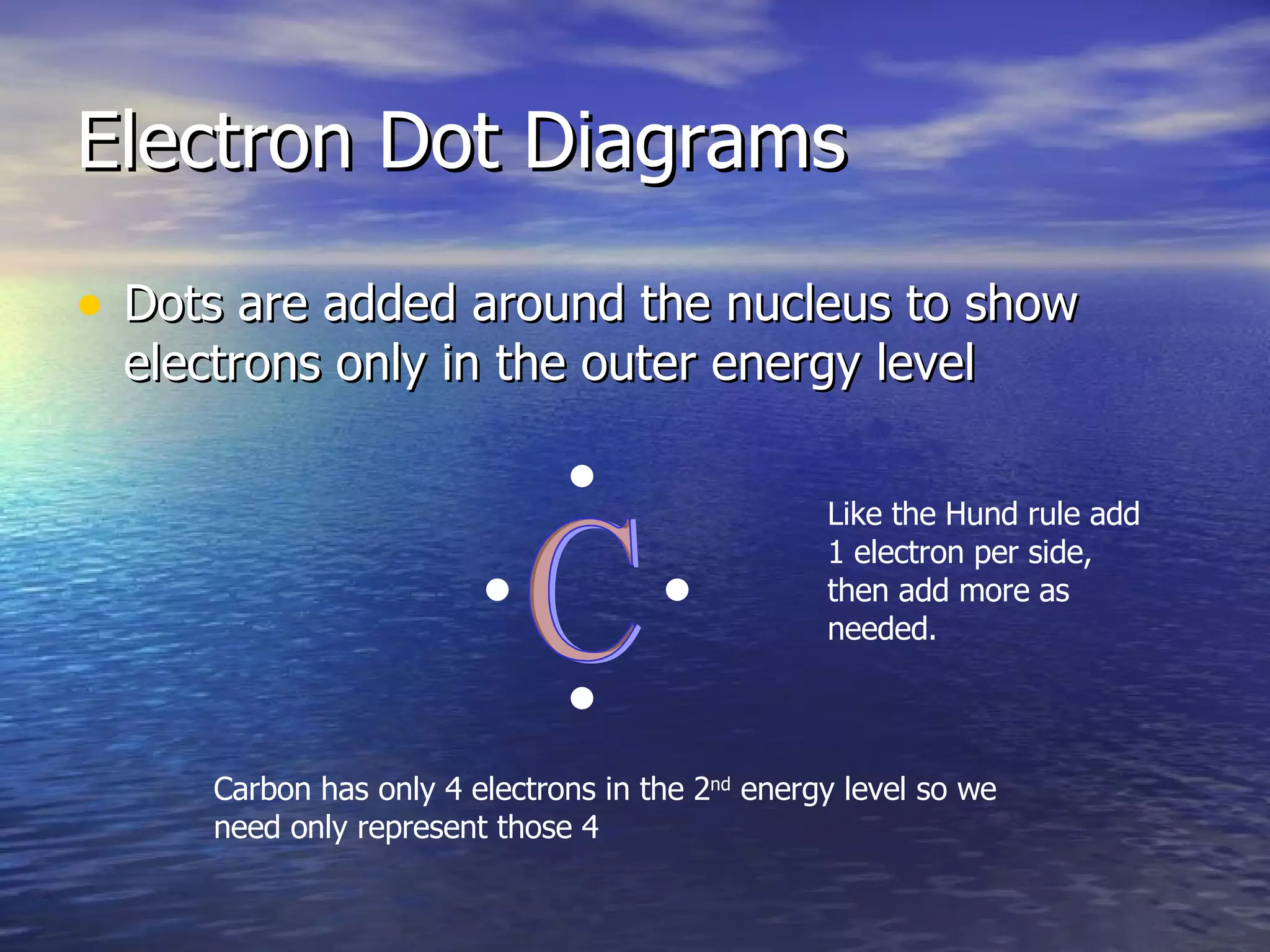
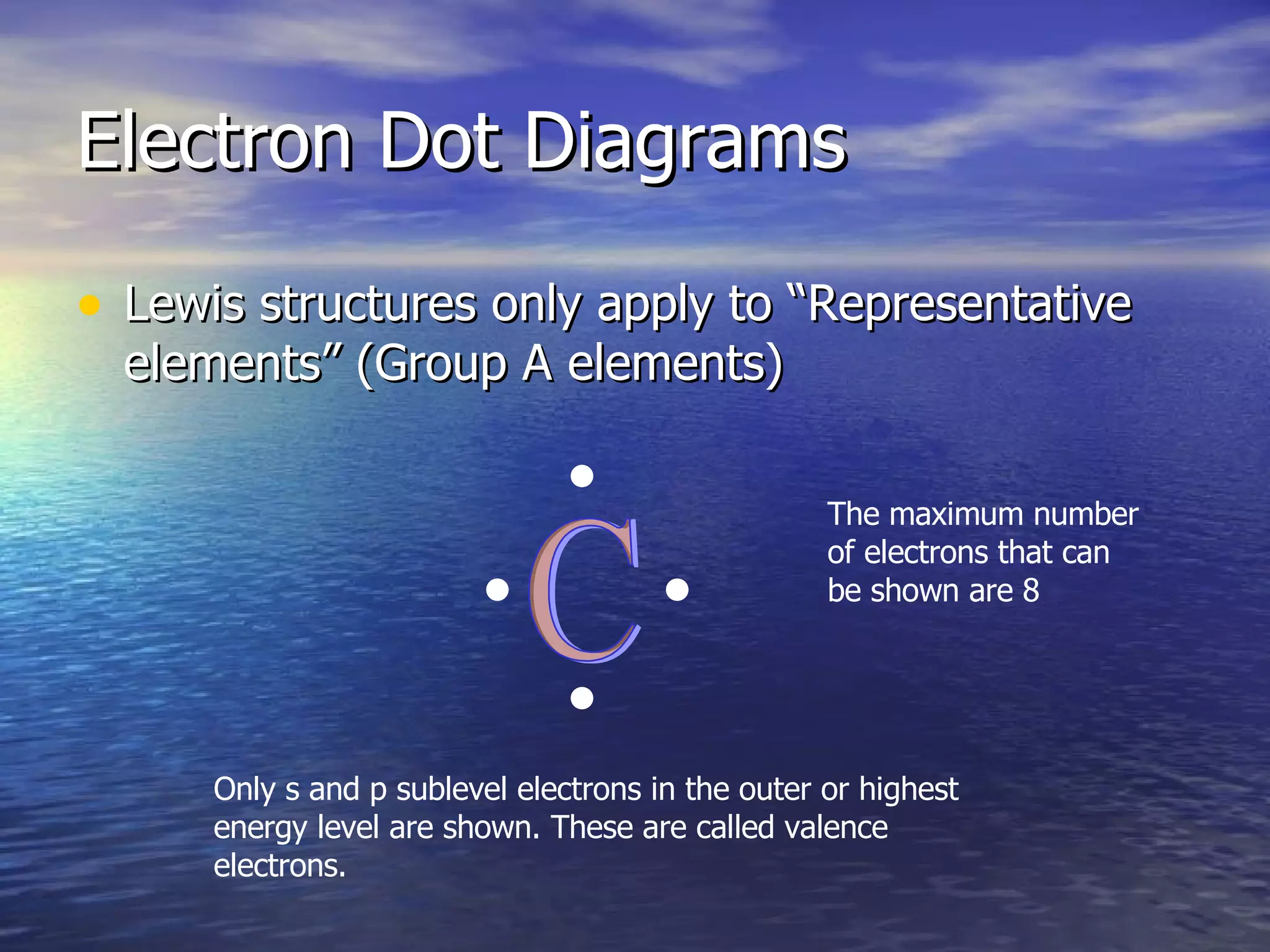
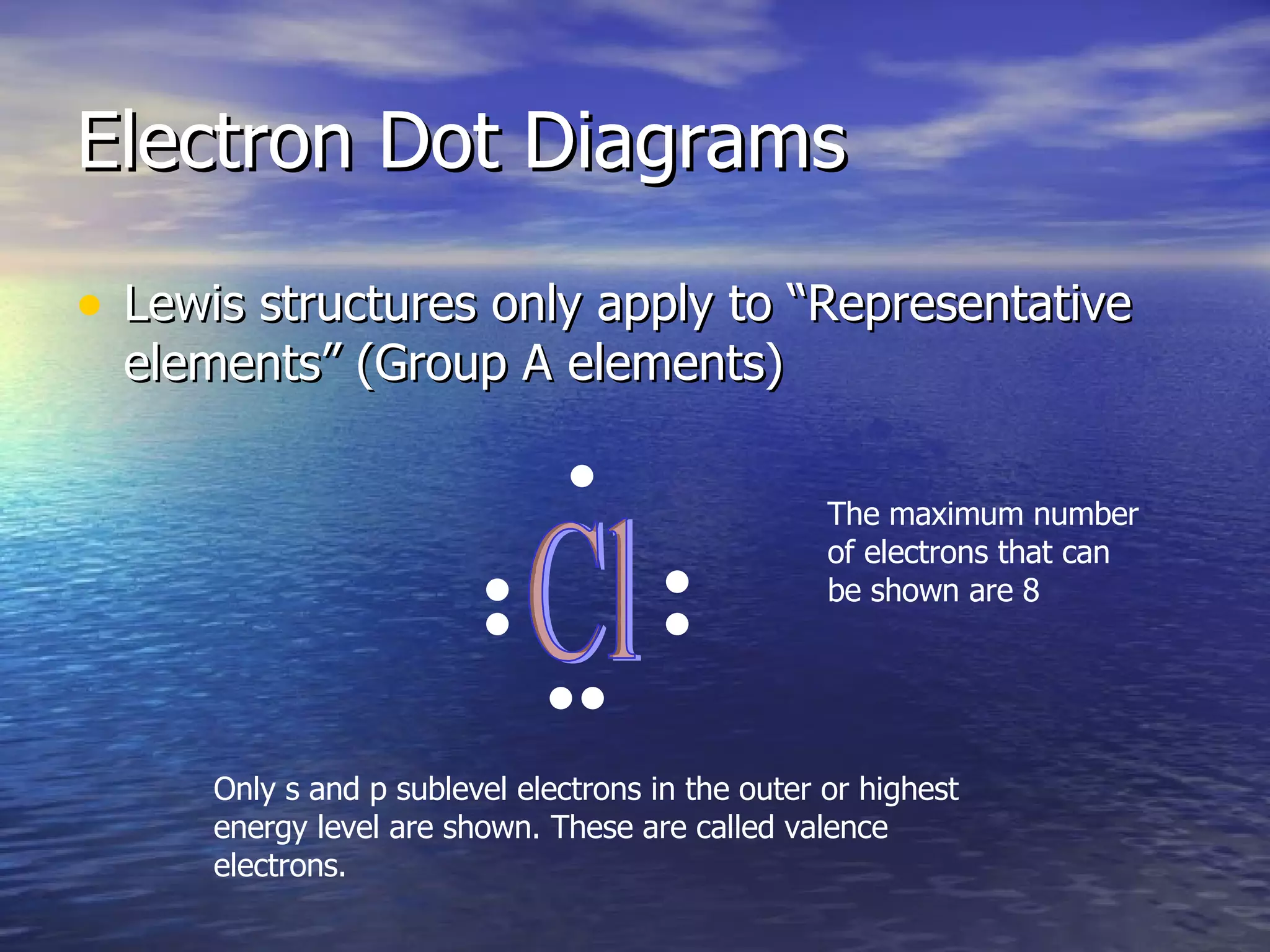
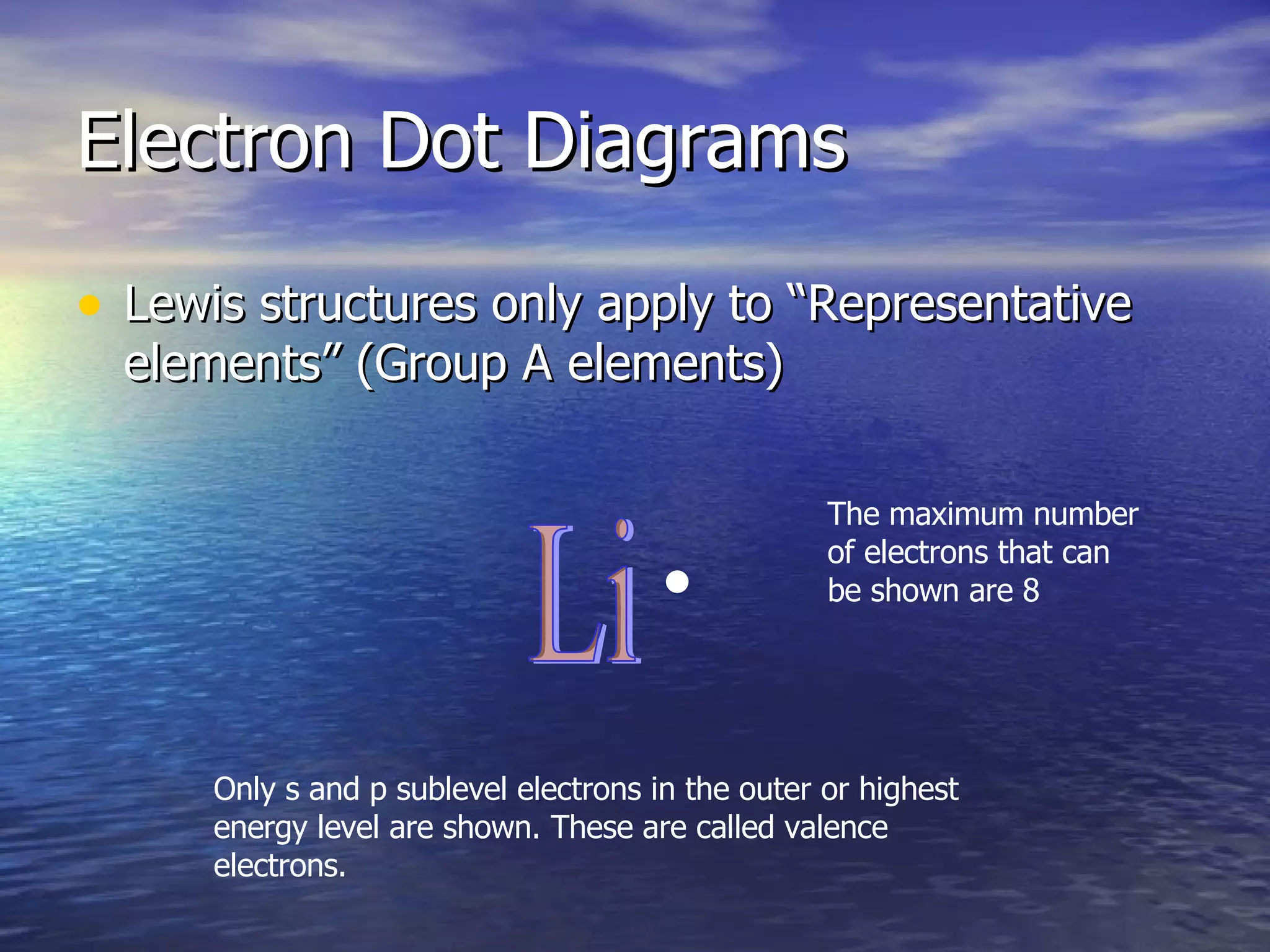
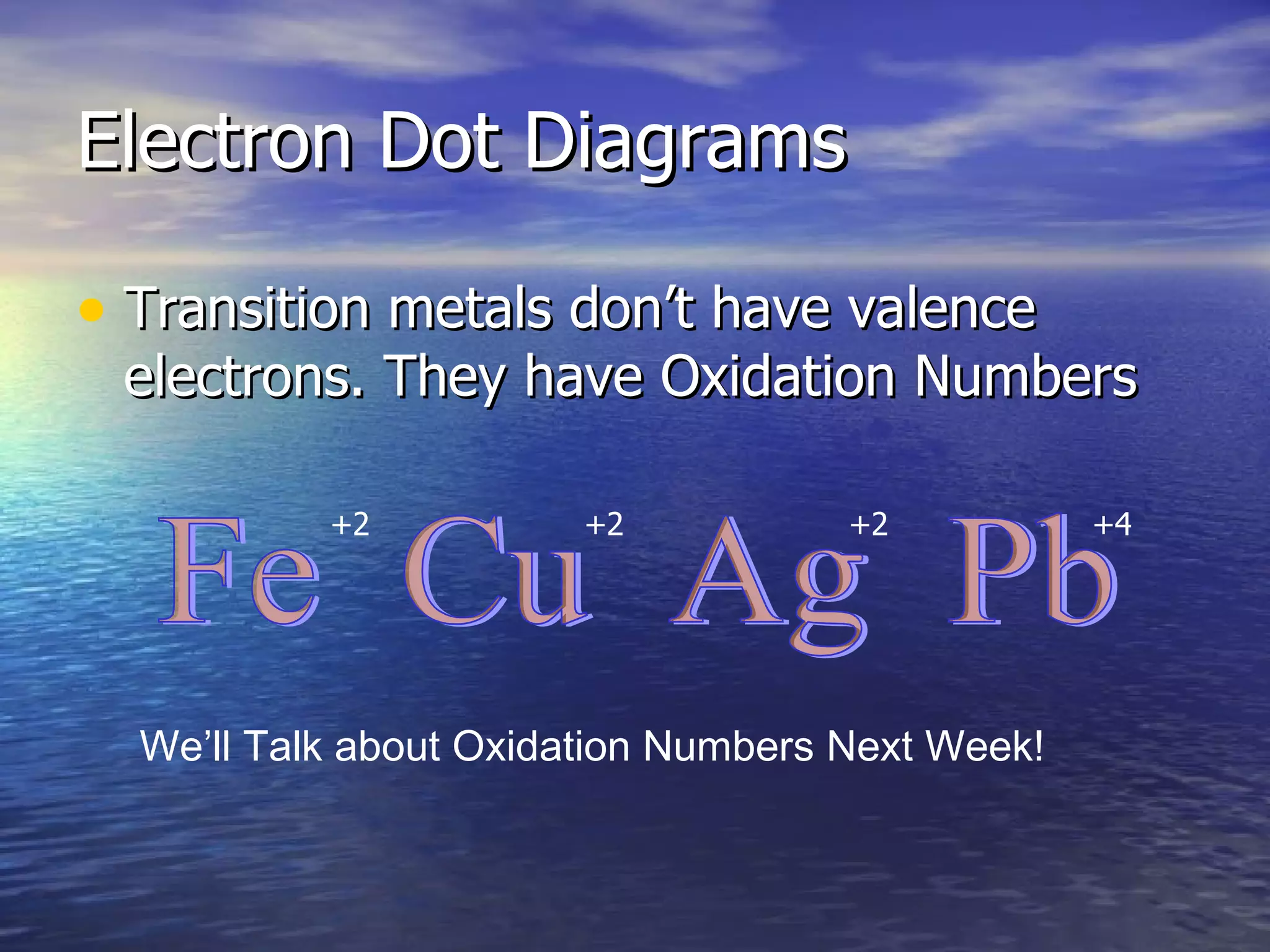
The document explains how to create Bohr diagrams and electron dot diagrams (Lewis structures) for atoms, specifically focusing on carbon as an example. Bohr diagrams illustrate the nucleus and electron energy levels, while electron dot diagrams represent only the valence electrons and are useful for understanding bonding geometry. Key points include the importance of accurately representing protons, neutrons, and electrons according to atomic number and mass.