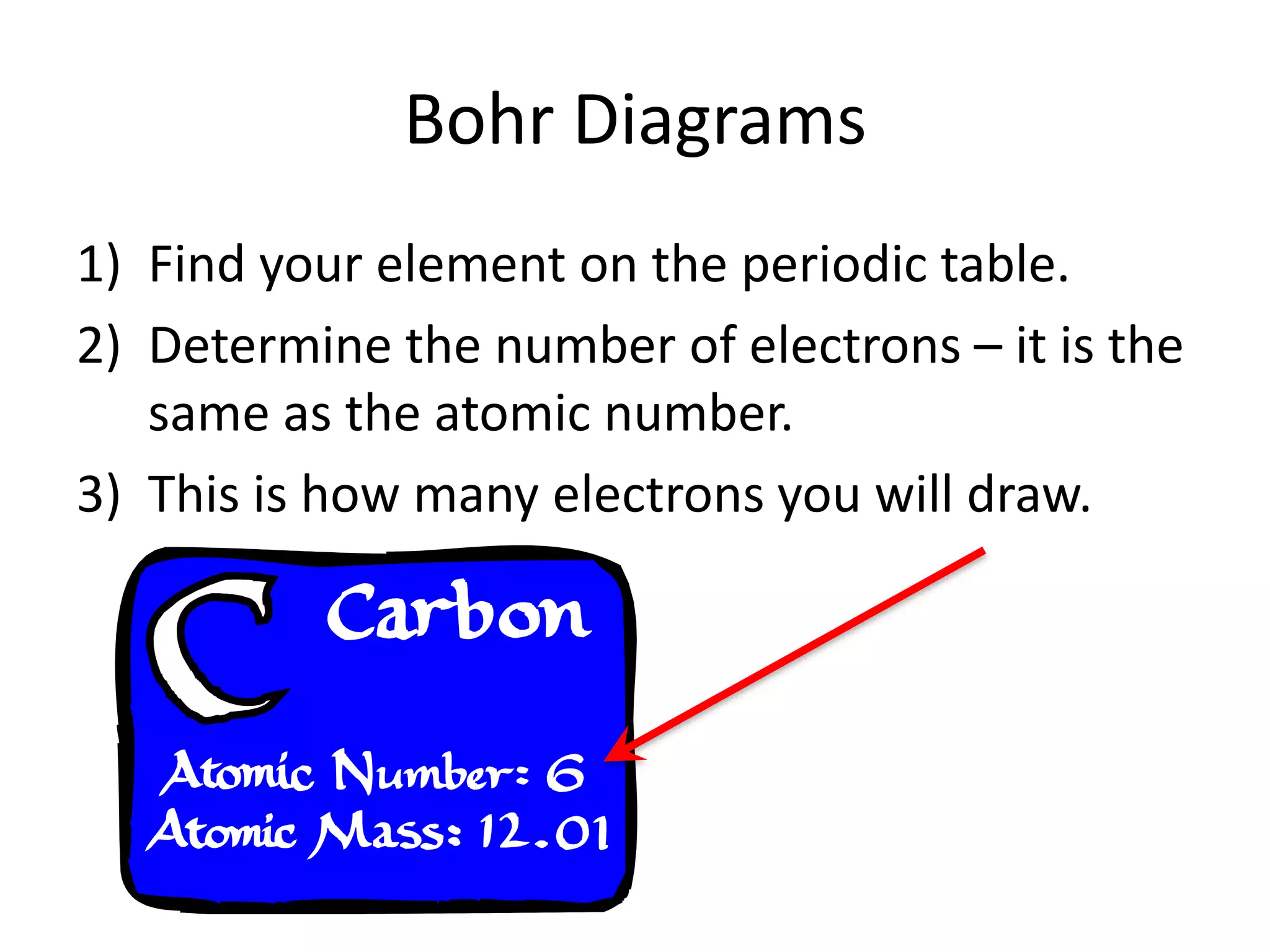
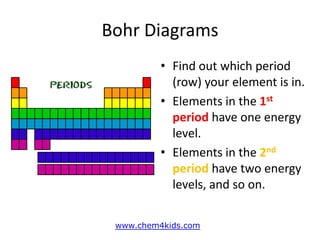
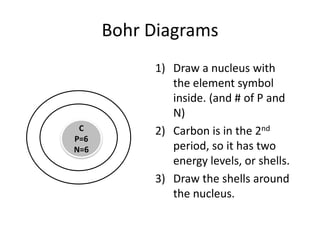
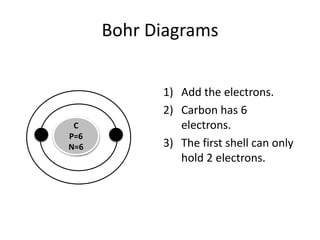
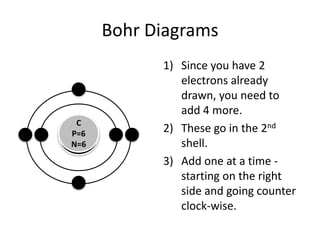
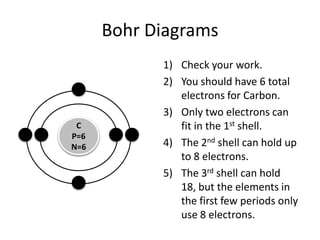
The document provides instructions for drawing Bohr diagrams to represent atomic structure. It explains that you determine the number of electrons from the element's atomic number and draw that number of electrons. It then describes how to draw the nucleus and electron shells based on the element's period, with inner shells able to hold a maximum of 2 or 8 electrons. The final diagram shows a completed Bohr diagram for carbon with its nucleus and two electron shells containing a total of 6 electrons.