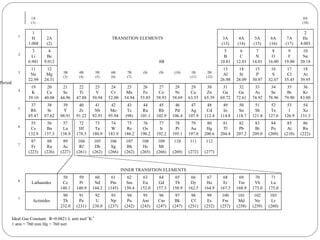
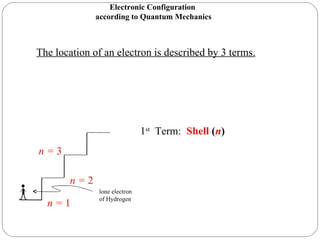
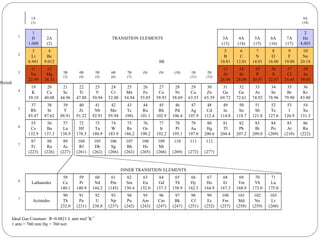
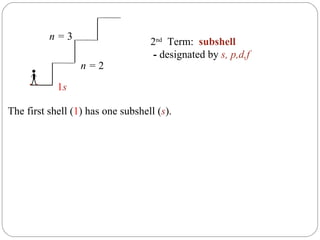
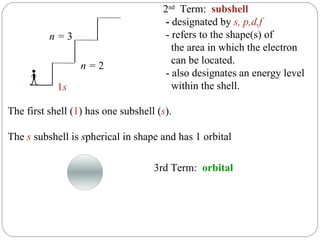
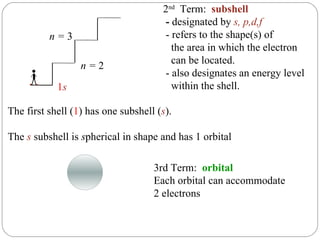
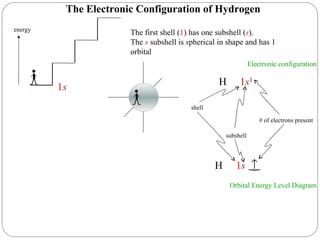
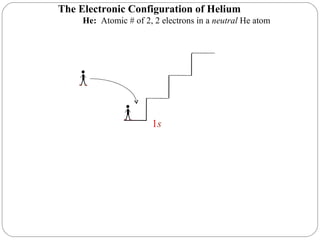
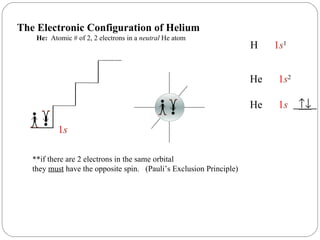
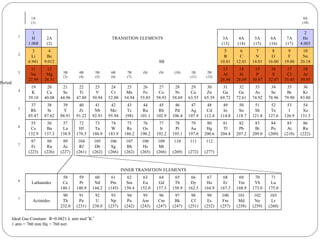
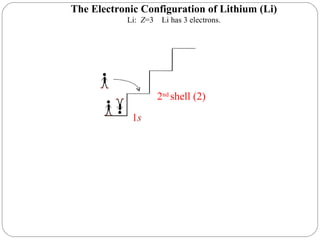
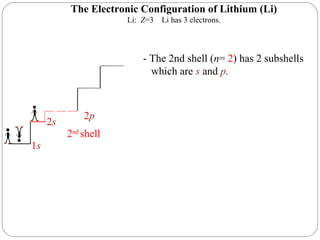
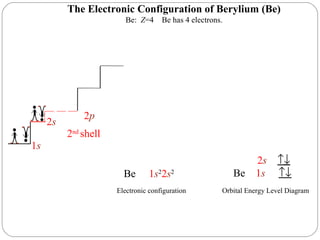
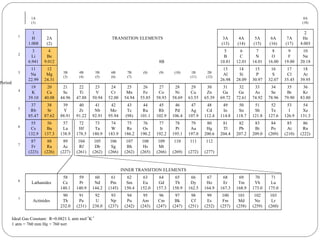
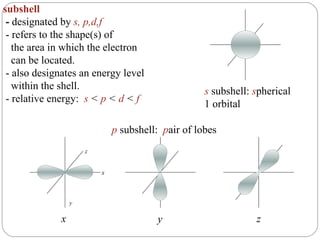
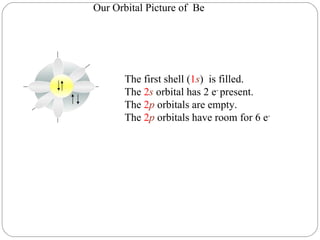
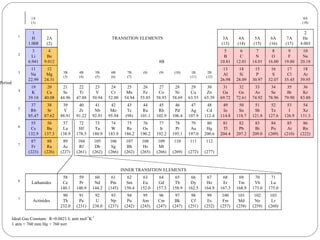
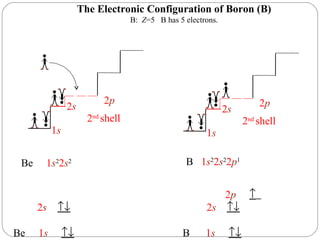
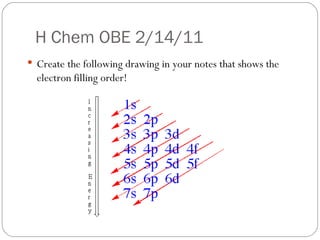
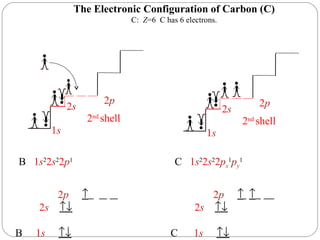
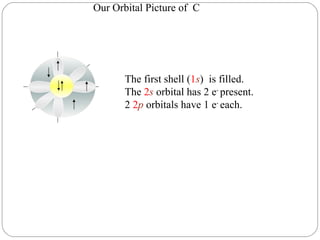
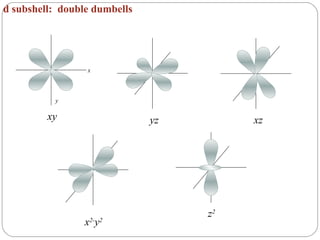
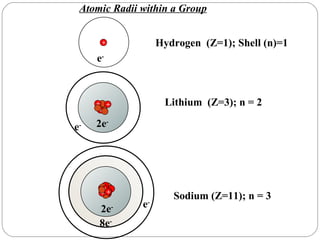
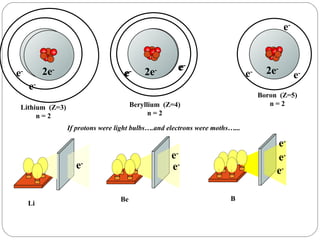
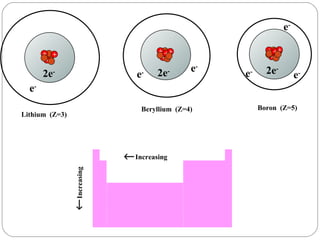
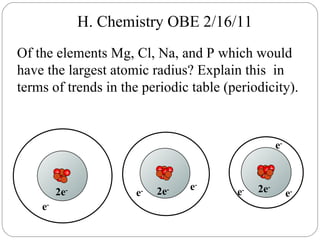
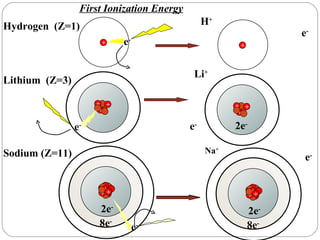
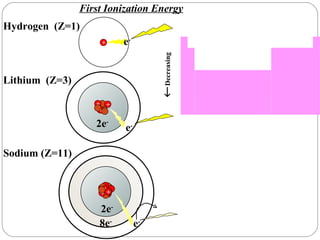
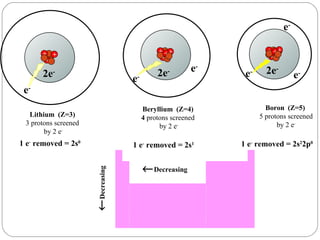
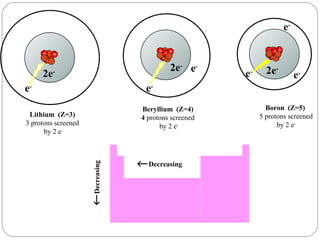
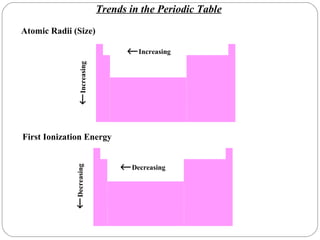
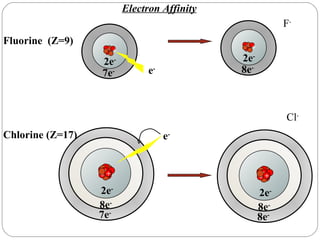
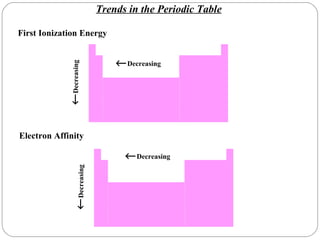
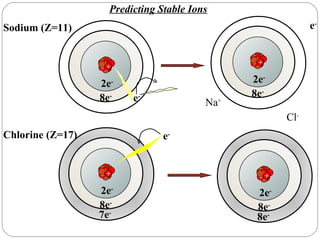
Rutherford discovered that the atom has a small, dense nucleus at its center, changing the old atomic model. Niels Bohr is most responsible for the current atomic model, which uses quantum mechanics to describe electrons orbiting the nucleus in shells and subshells based on their energy levels. Electrons fill these orbitals according to specific rules.