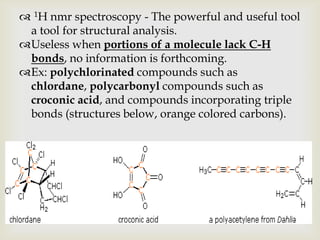
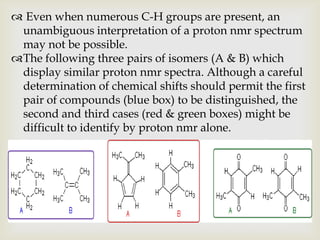
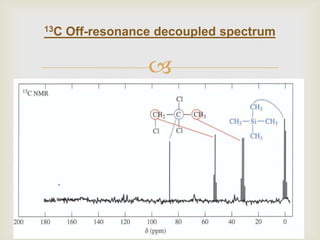
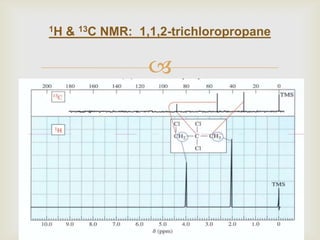
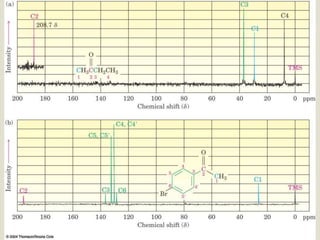
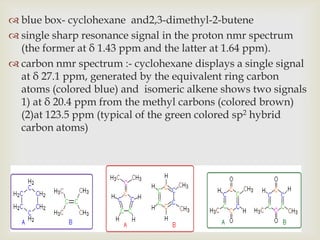
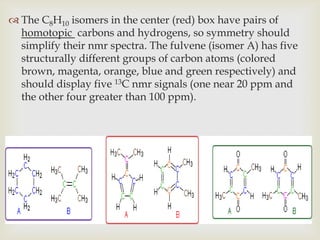
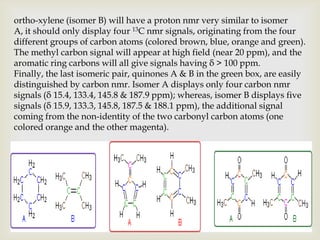
1) 13C NMR spectroscopy provides valuable structural information when 1H NMR is insufficient or ambiguous. It directly detects carbon atoms and gives signals based on their chemical environment rather than hydrogen bonding.
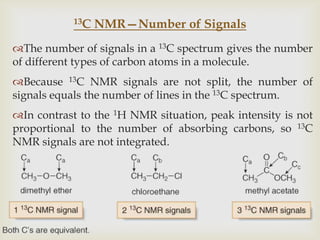
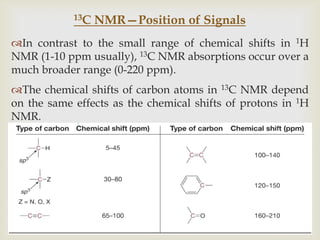
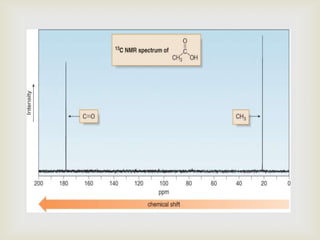
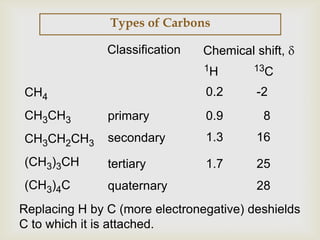
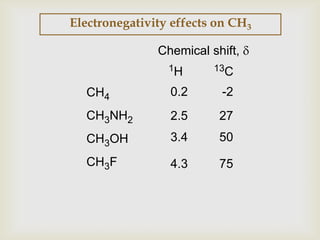
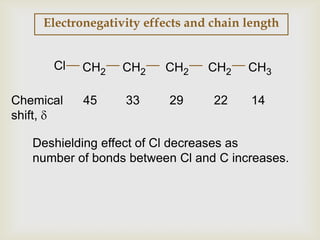
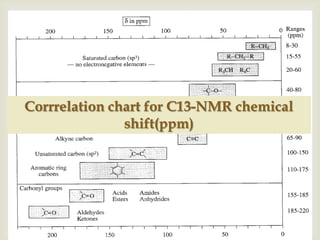
2) 13C NMR spectra contain information about the number and types of carbon atoms present based on the number of signals and their chemical shifts. The chemical shifts are influenced by factors like hybridization and electronegativity.
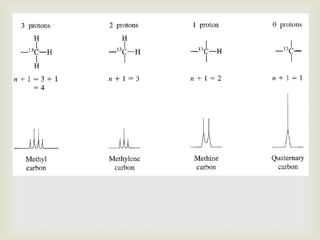
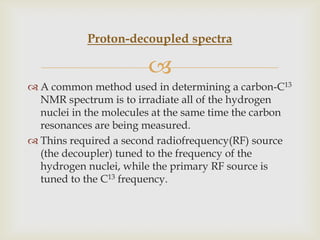
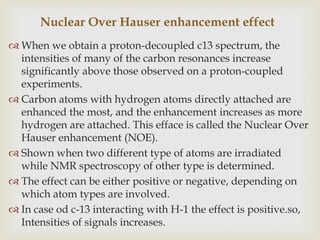
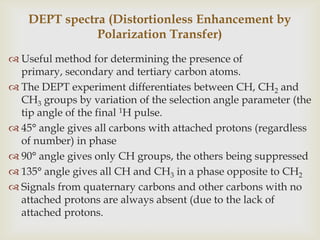
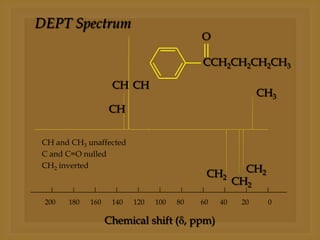
3) Techniques like proton decoupling and DEPT allow differentiation of carbon types like CH, CH2, and CH3 based on their signal behavior under different pulse sequences.