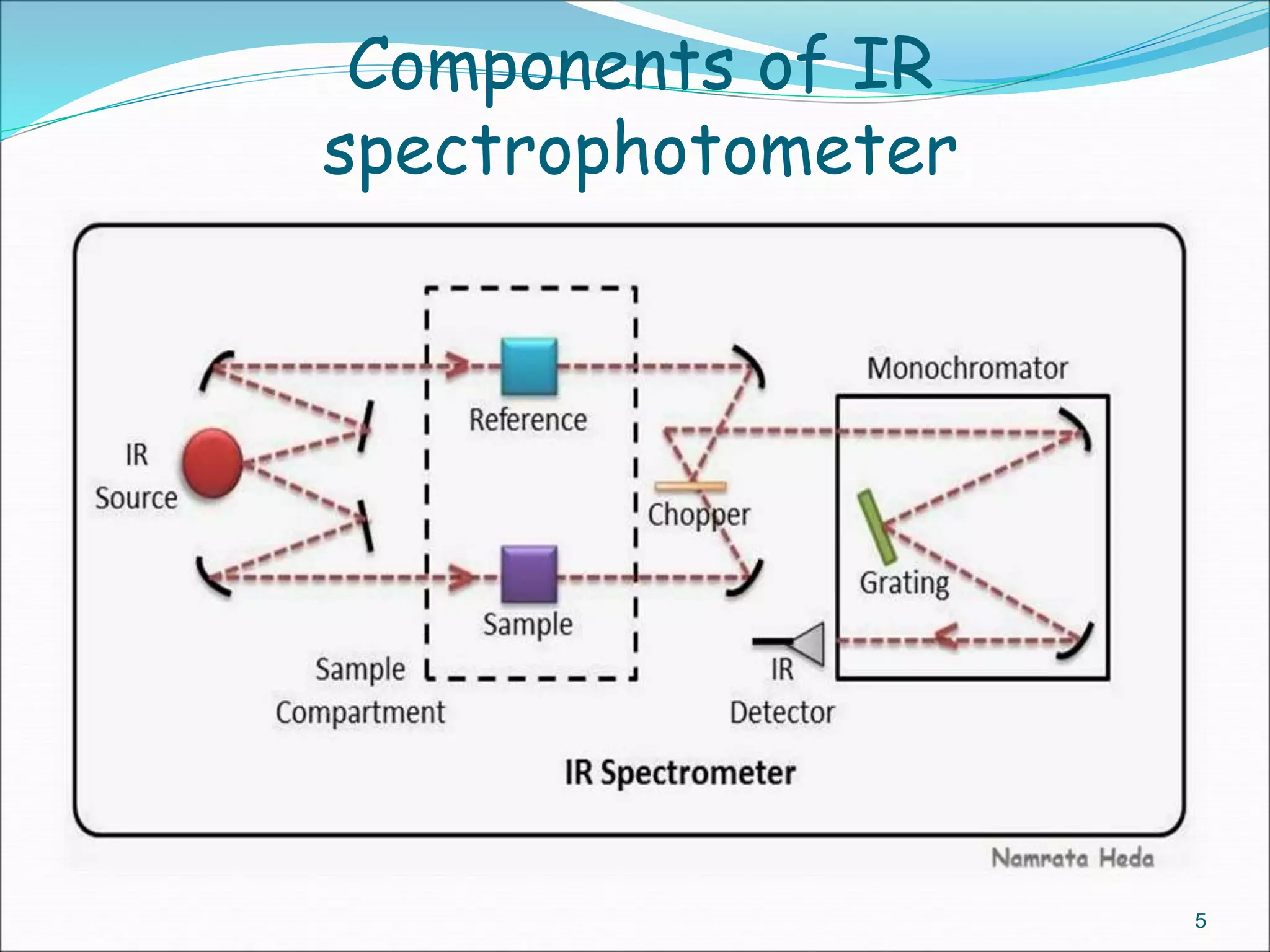
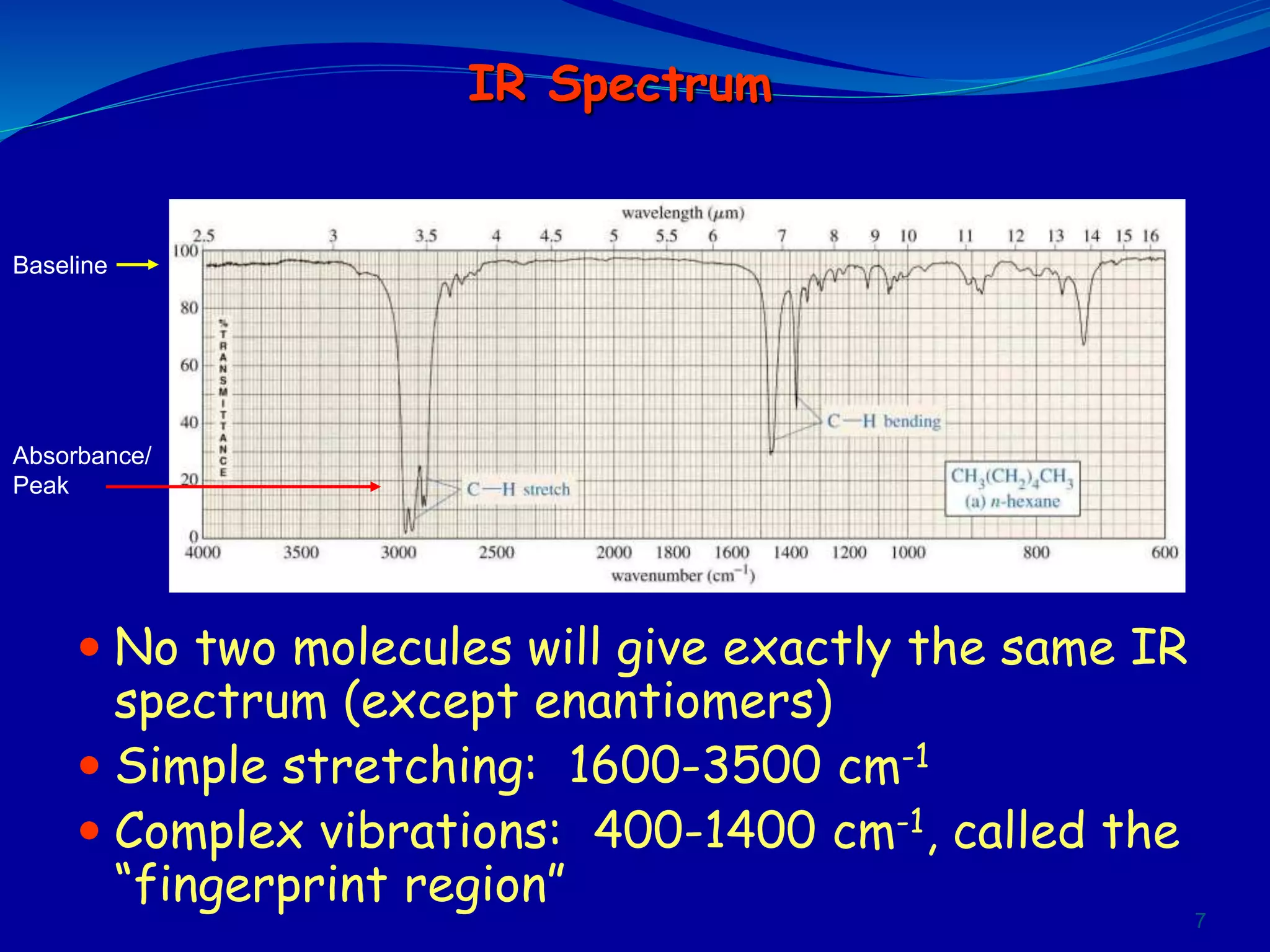
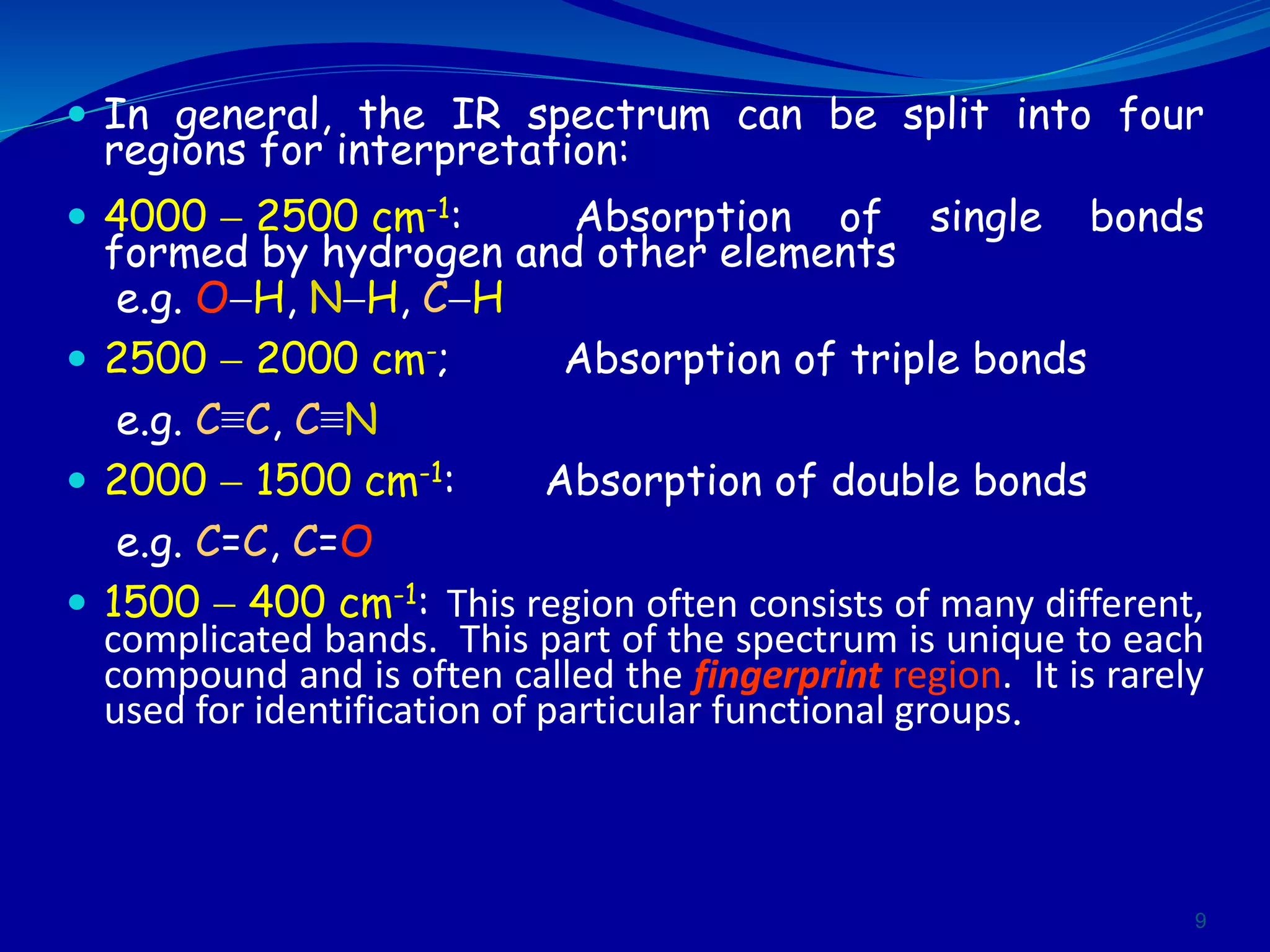
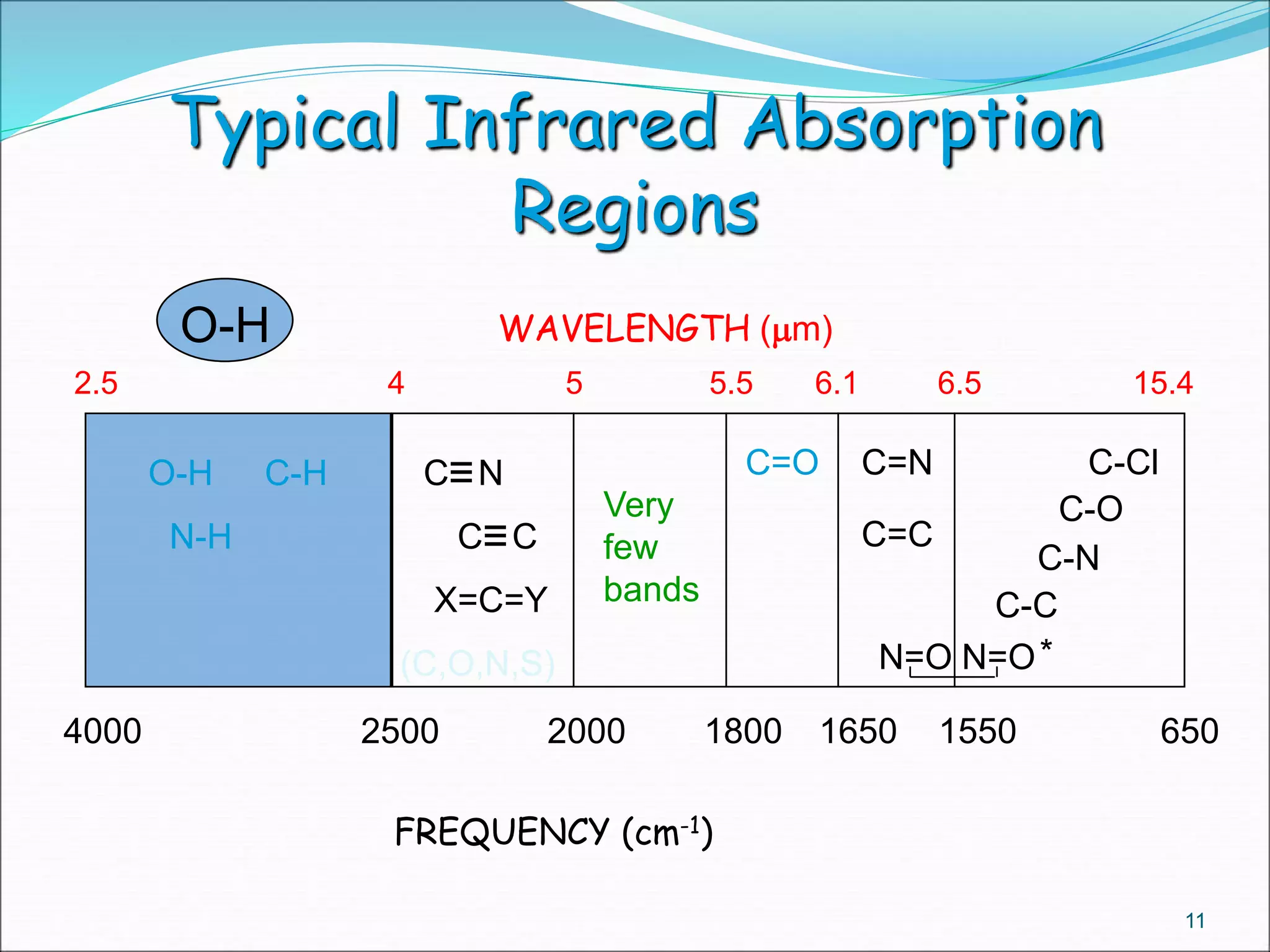
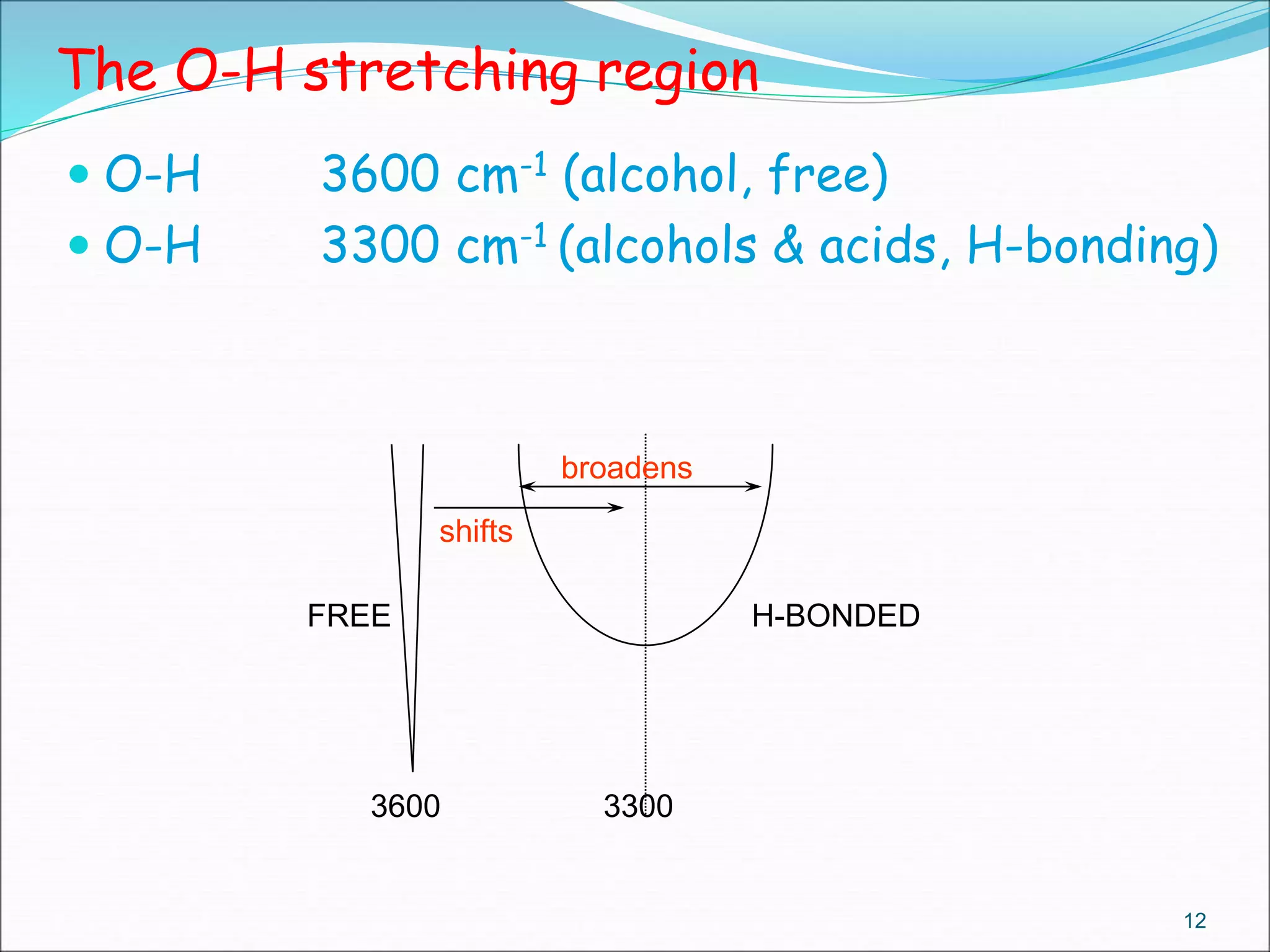
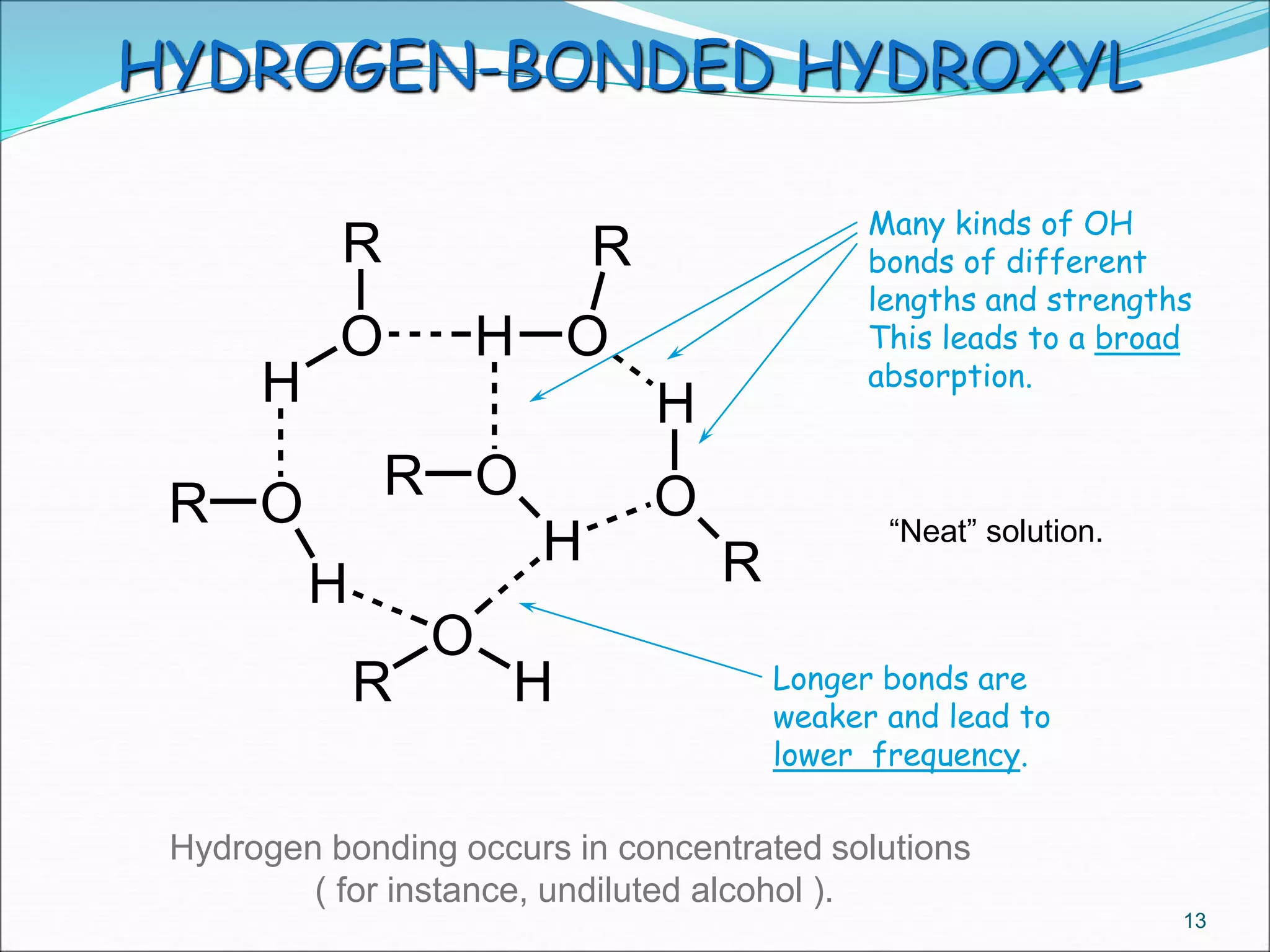
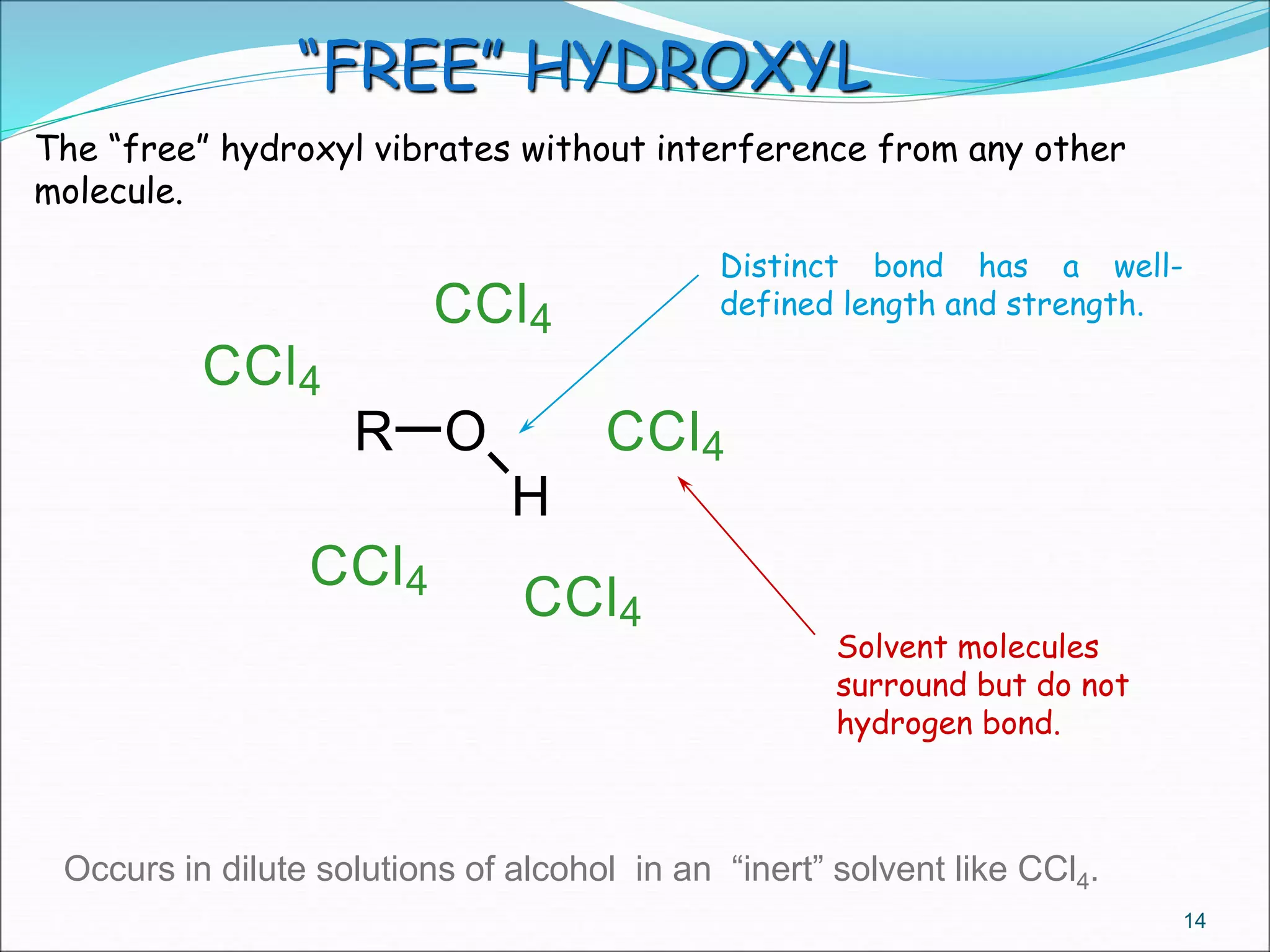
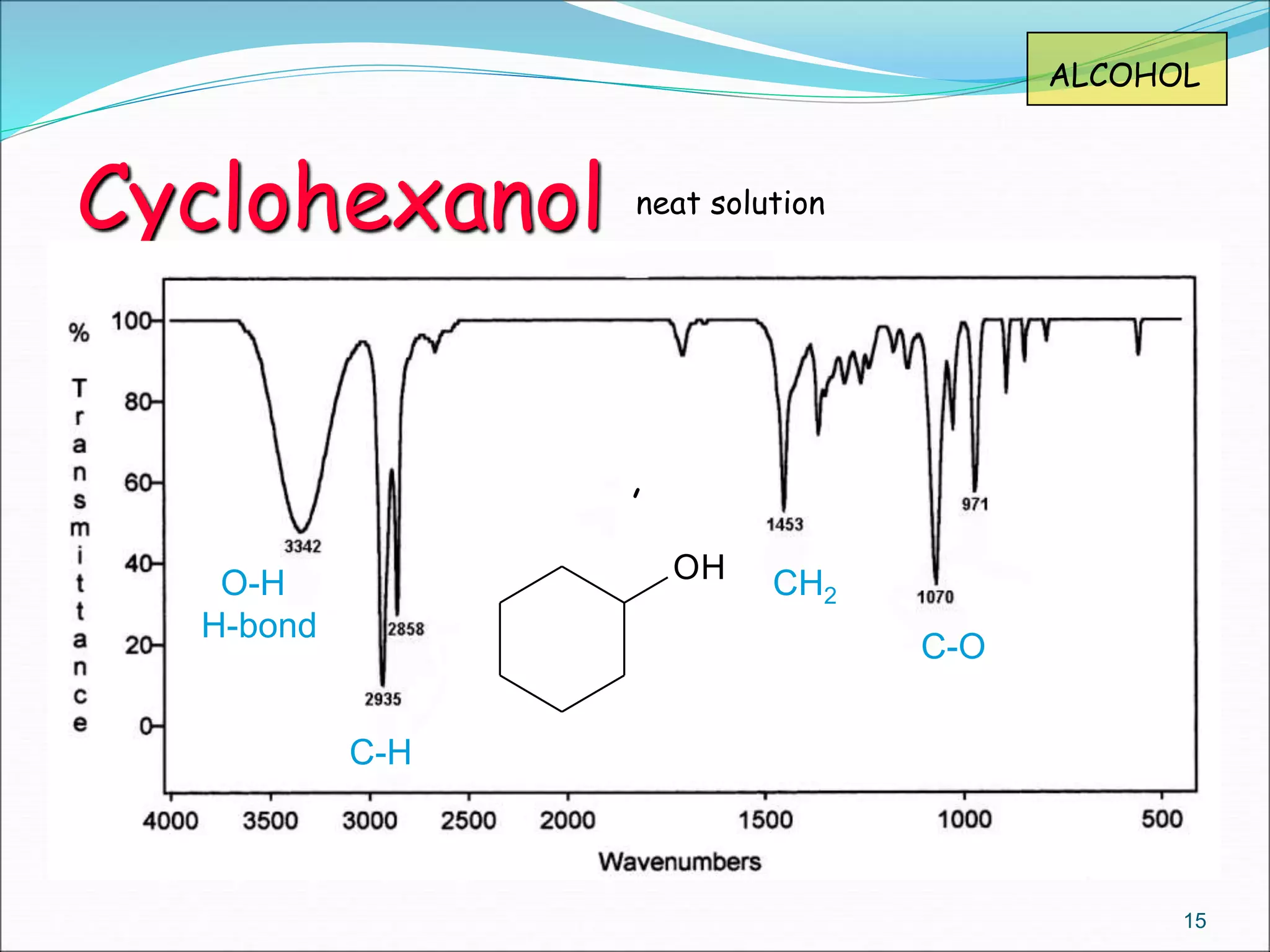
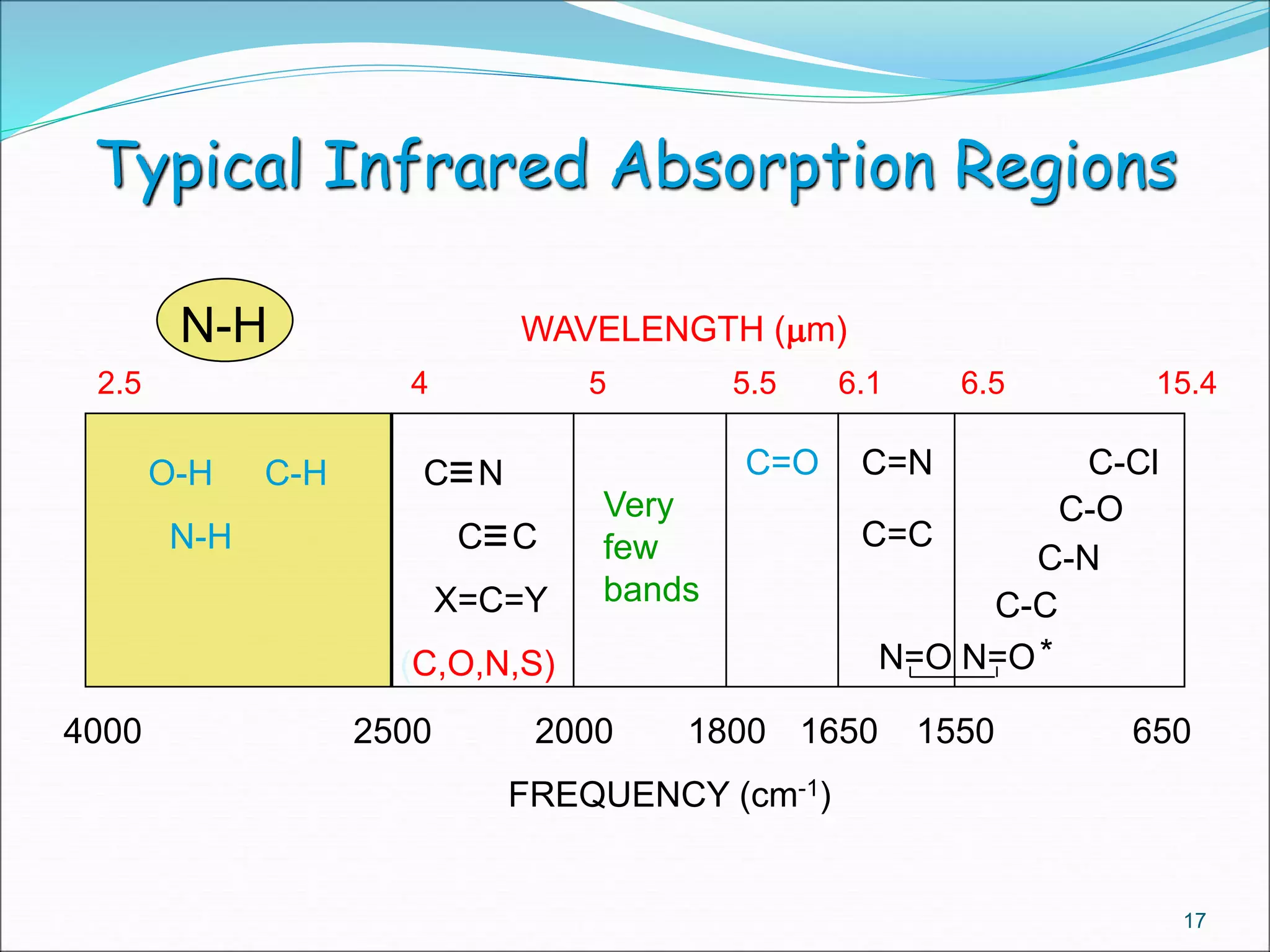
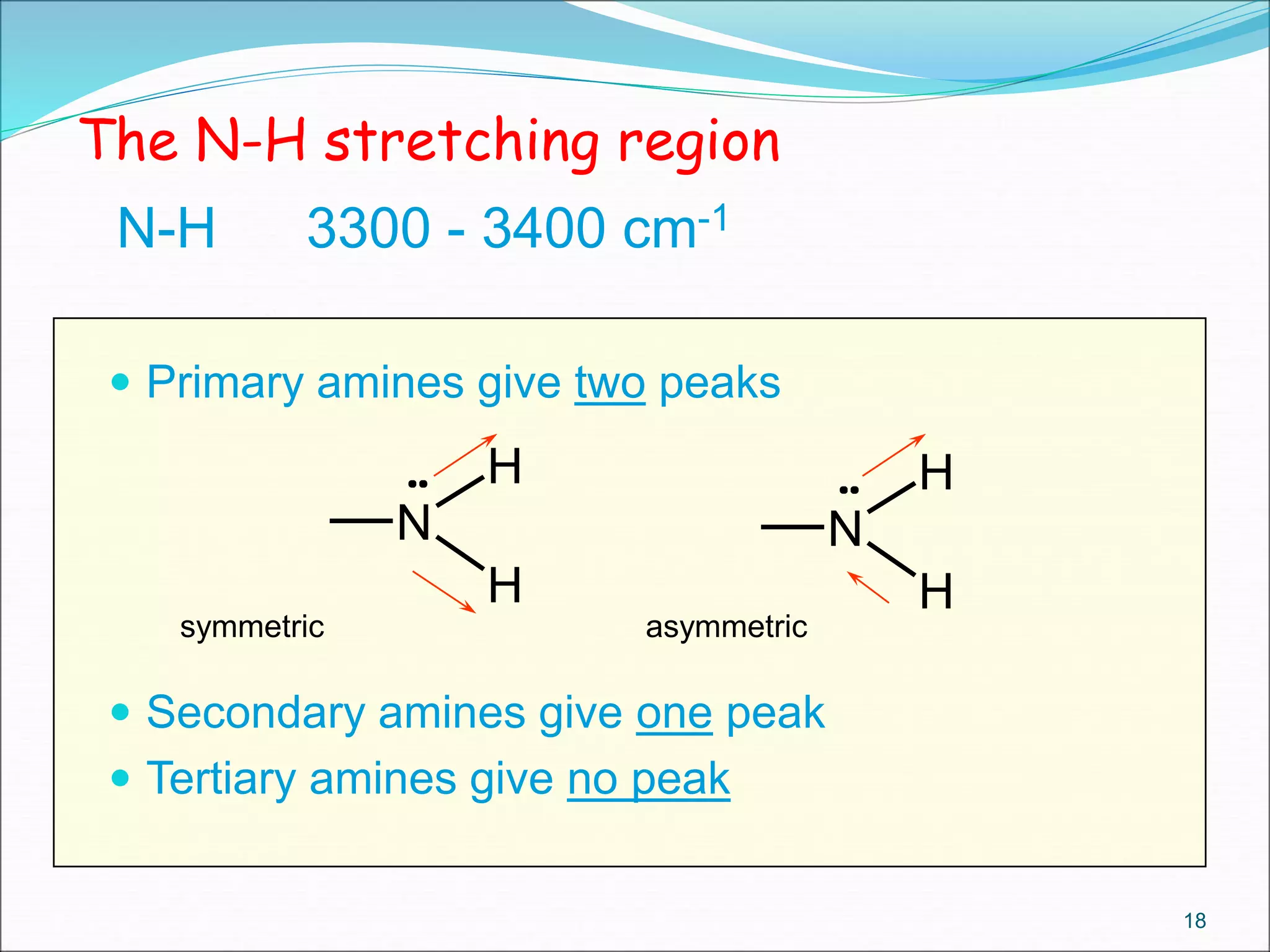
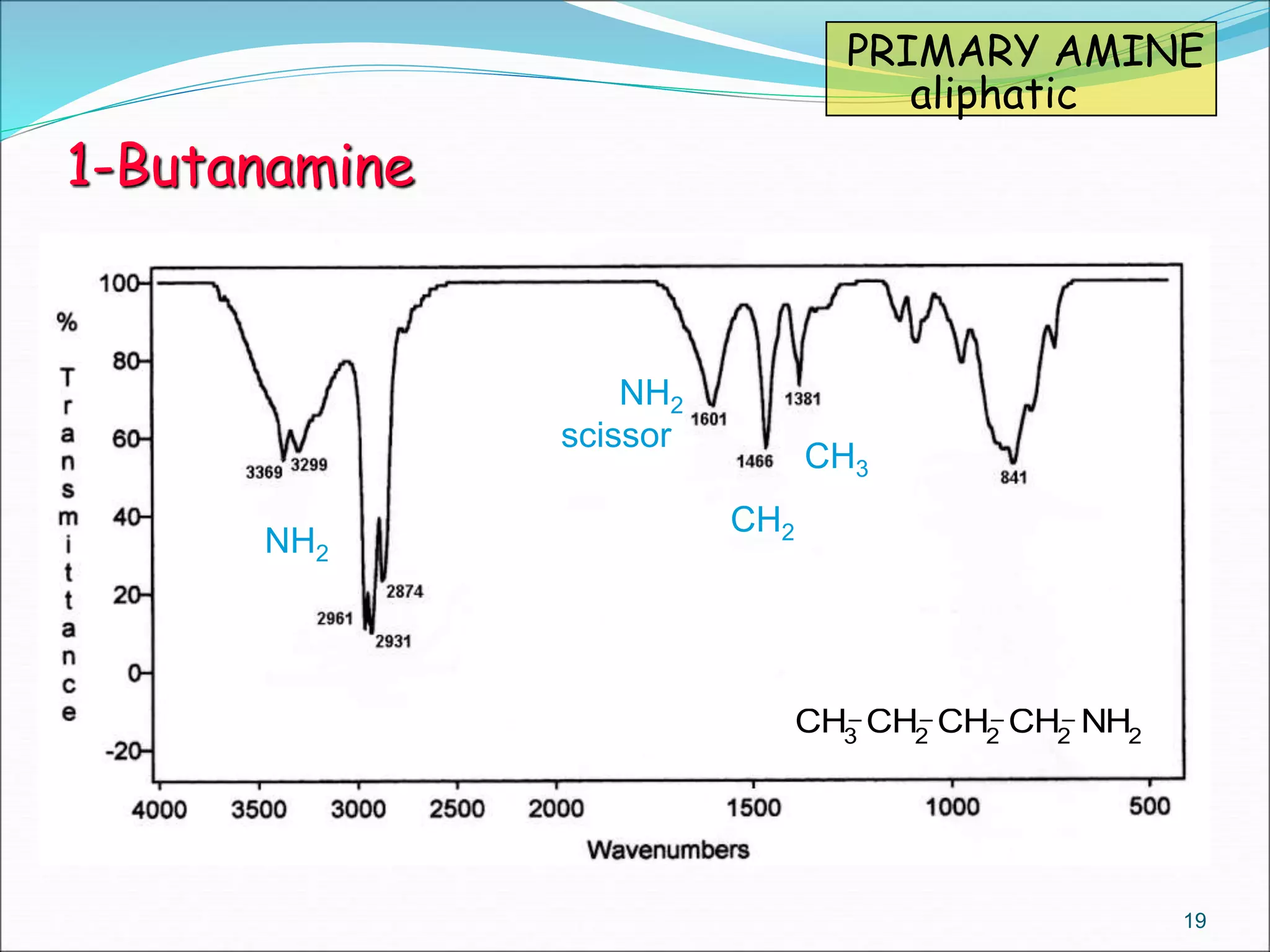
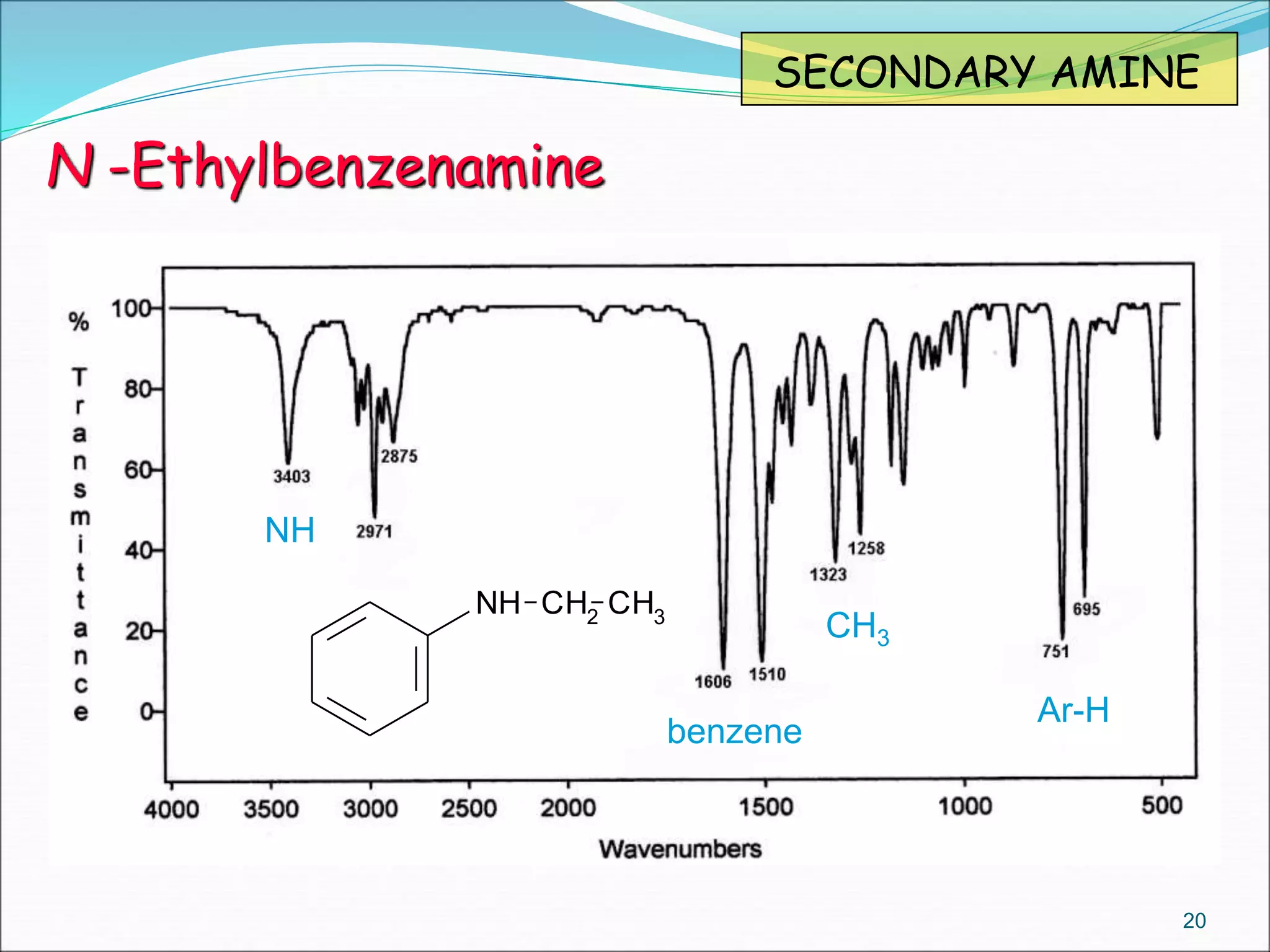
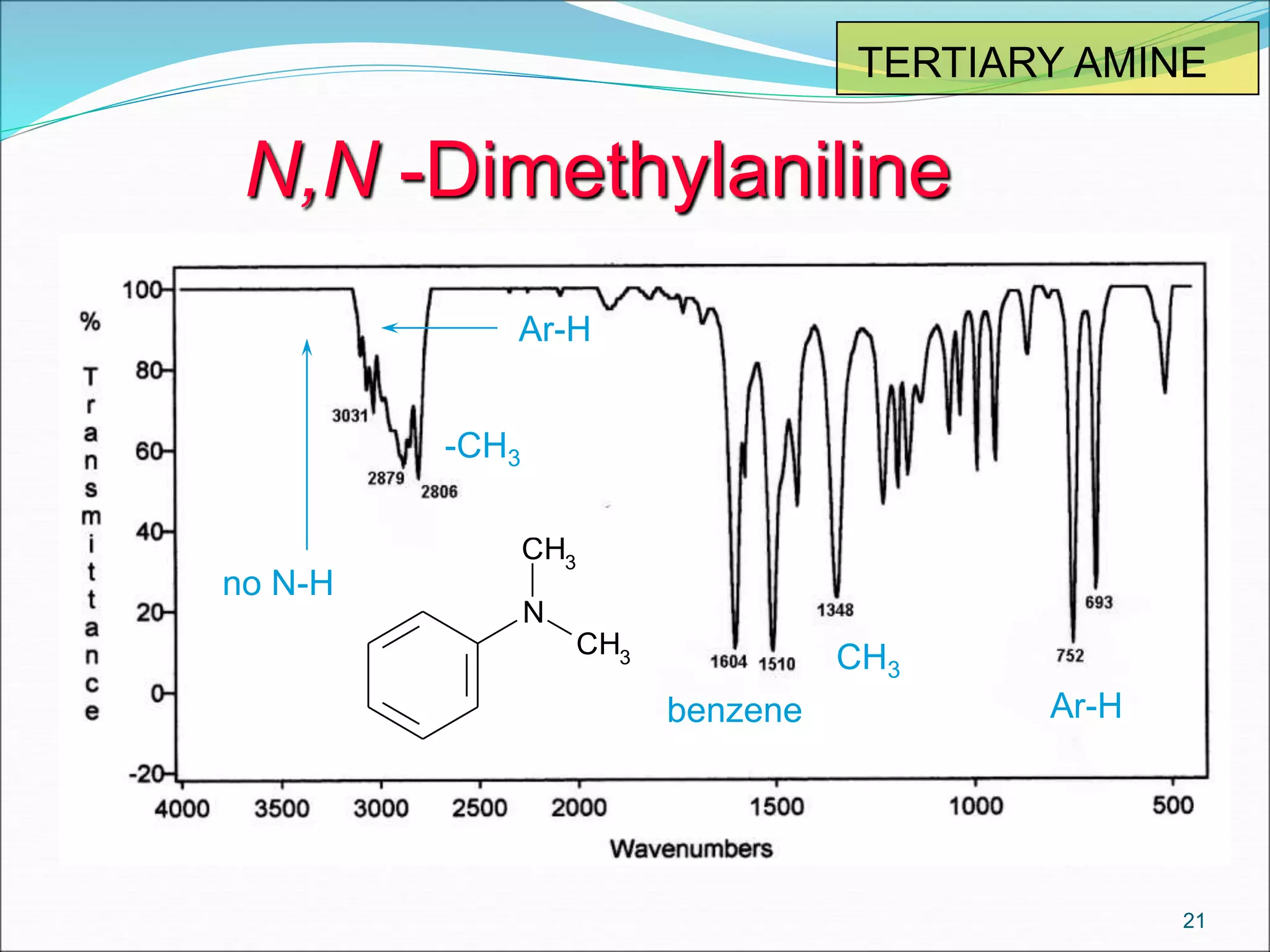
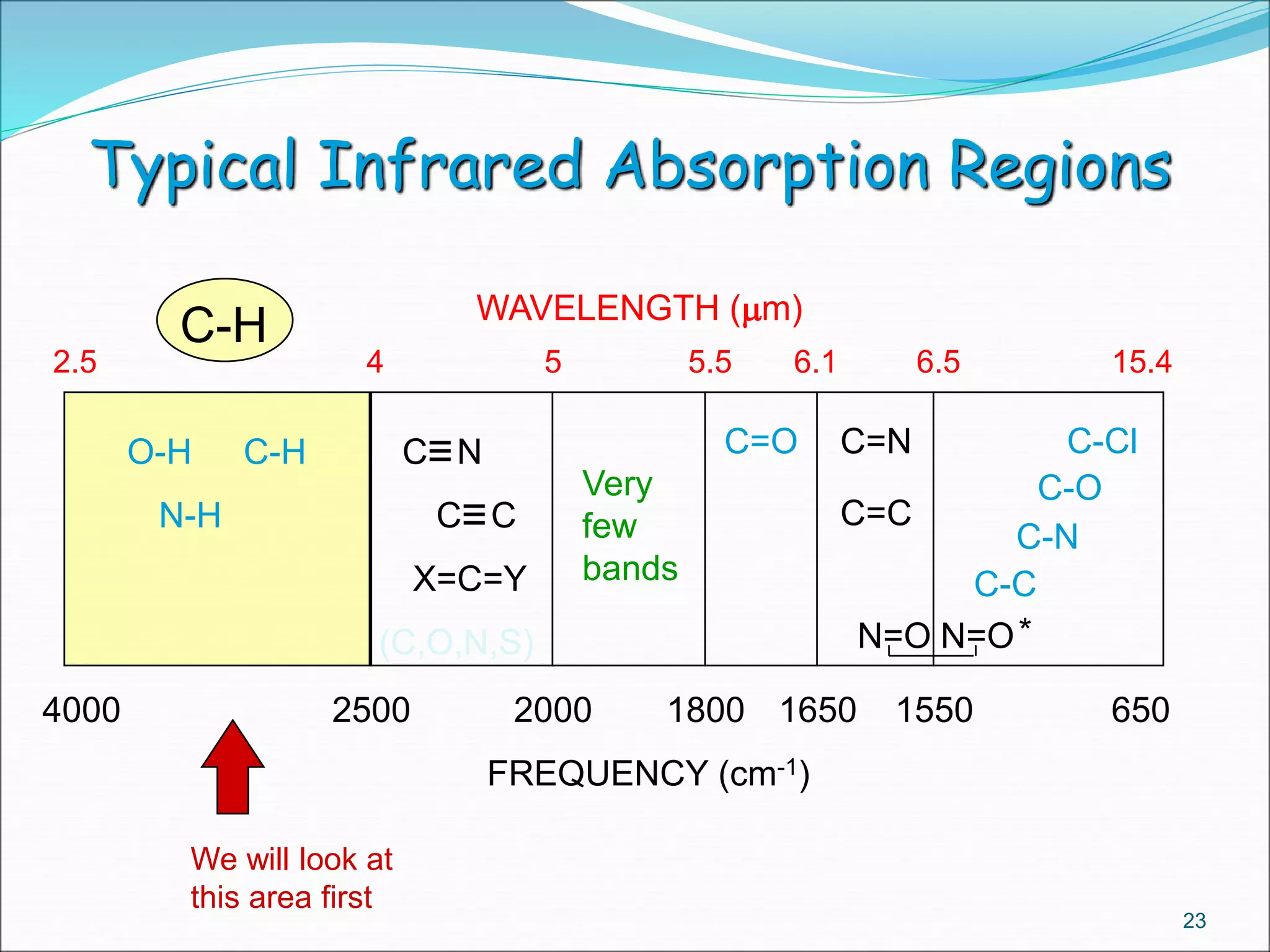
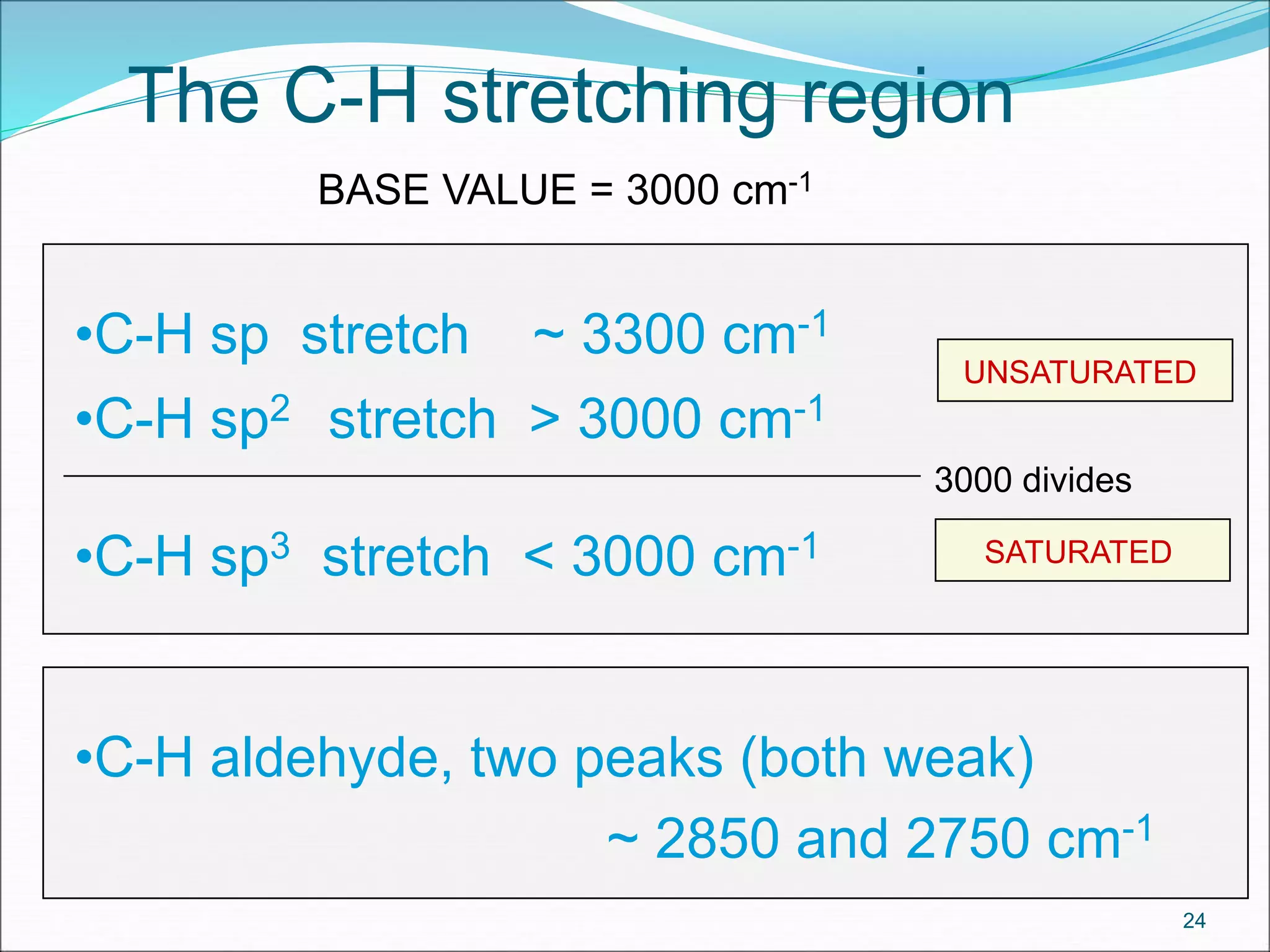
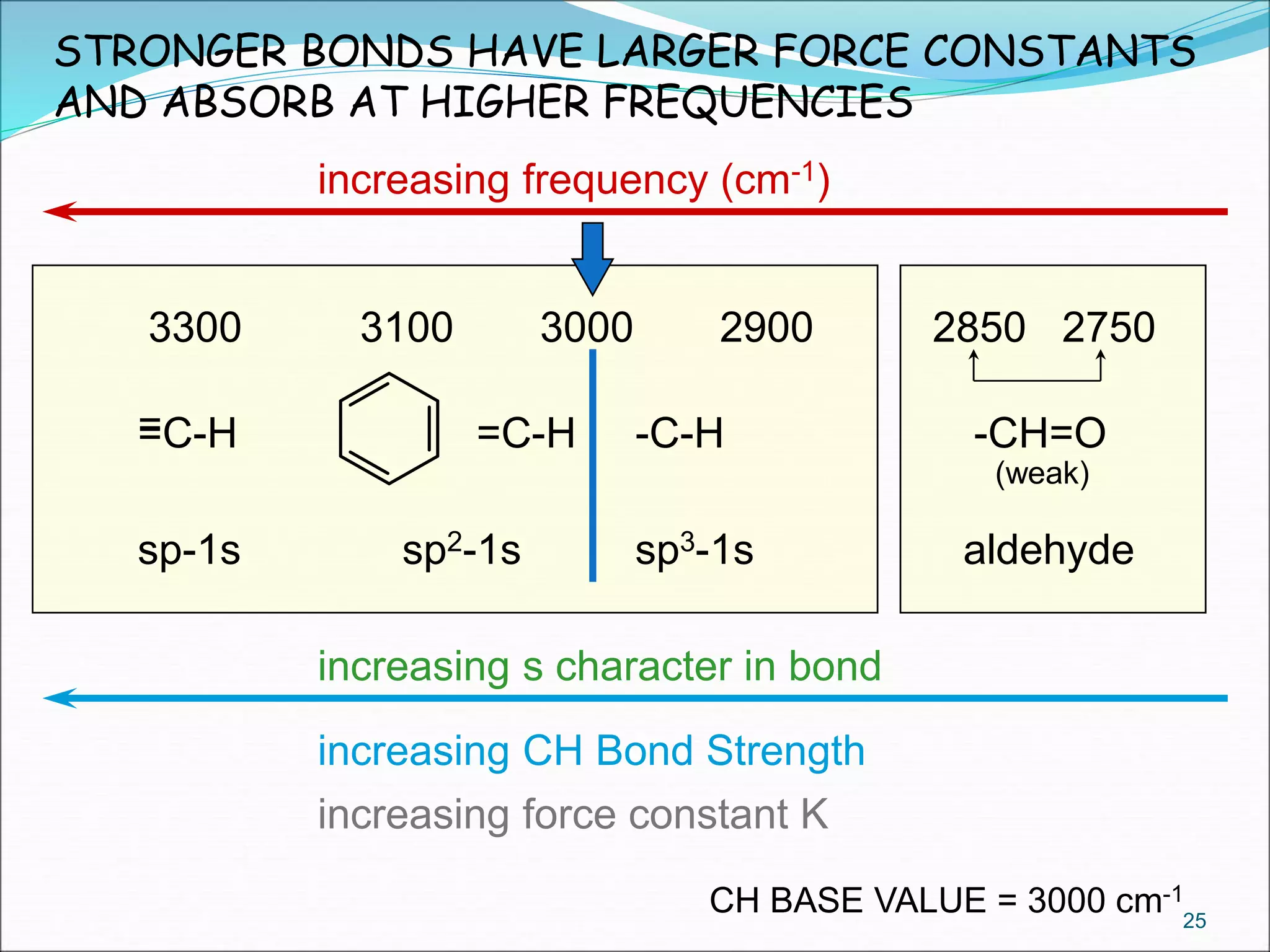
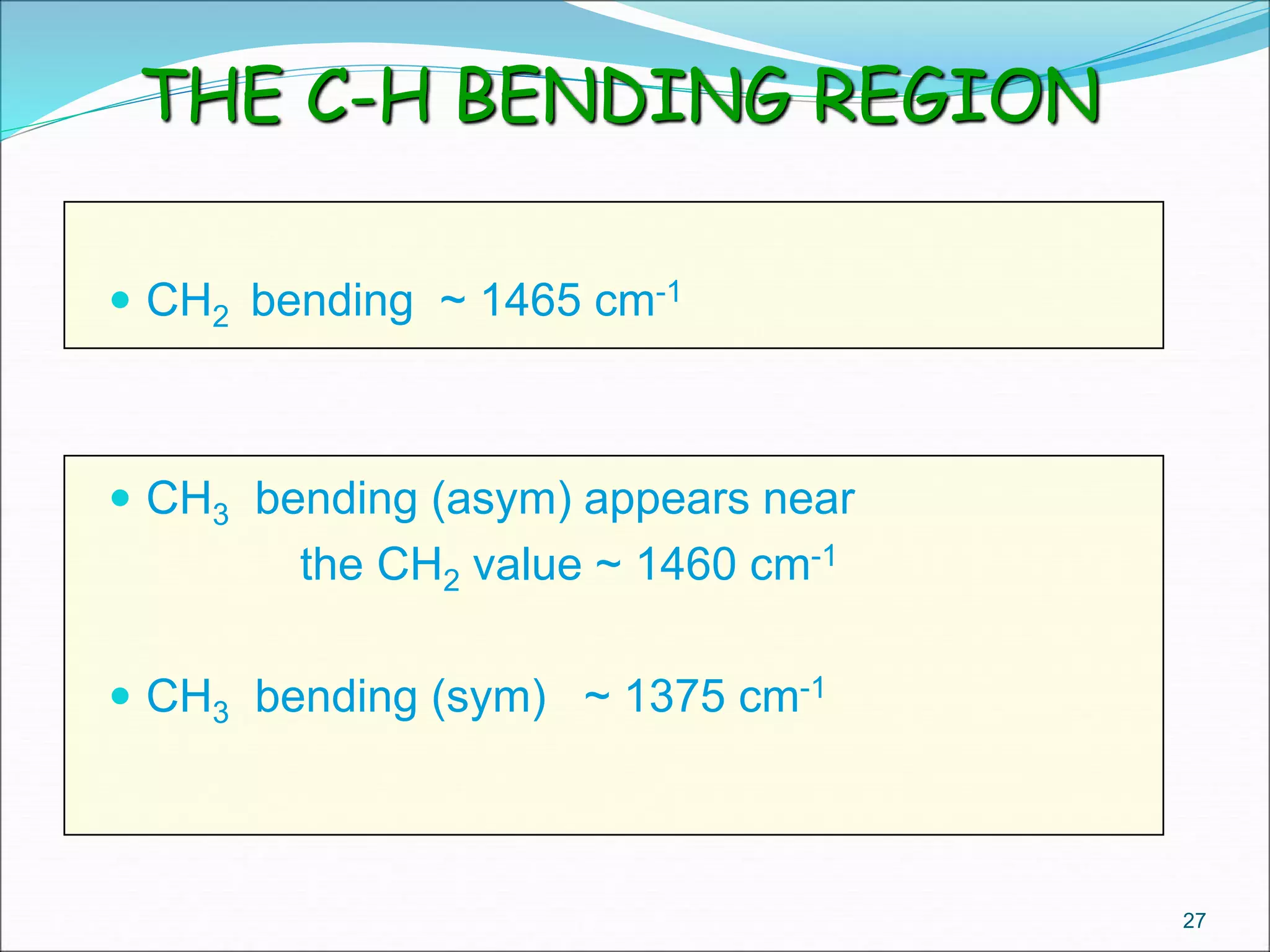
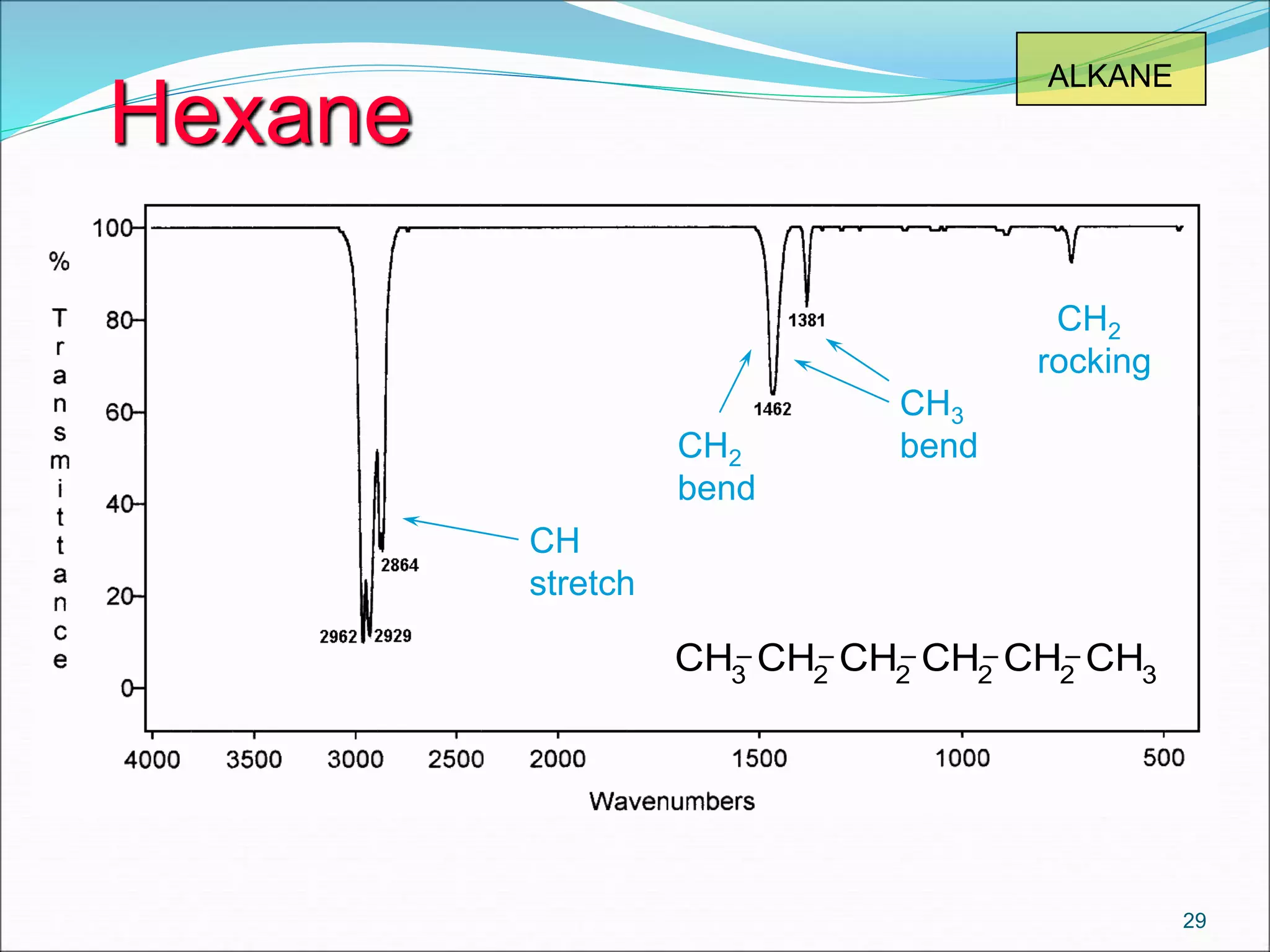
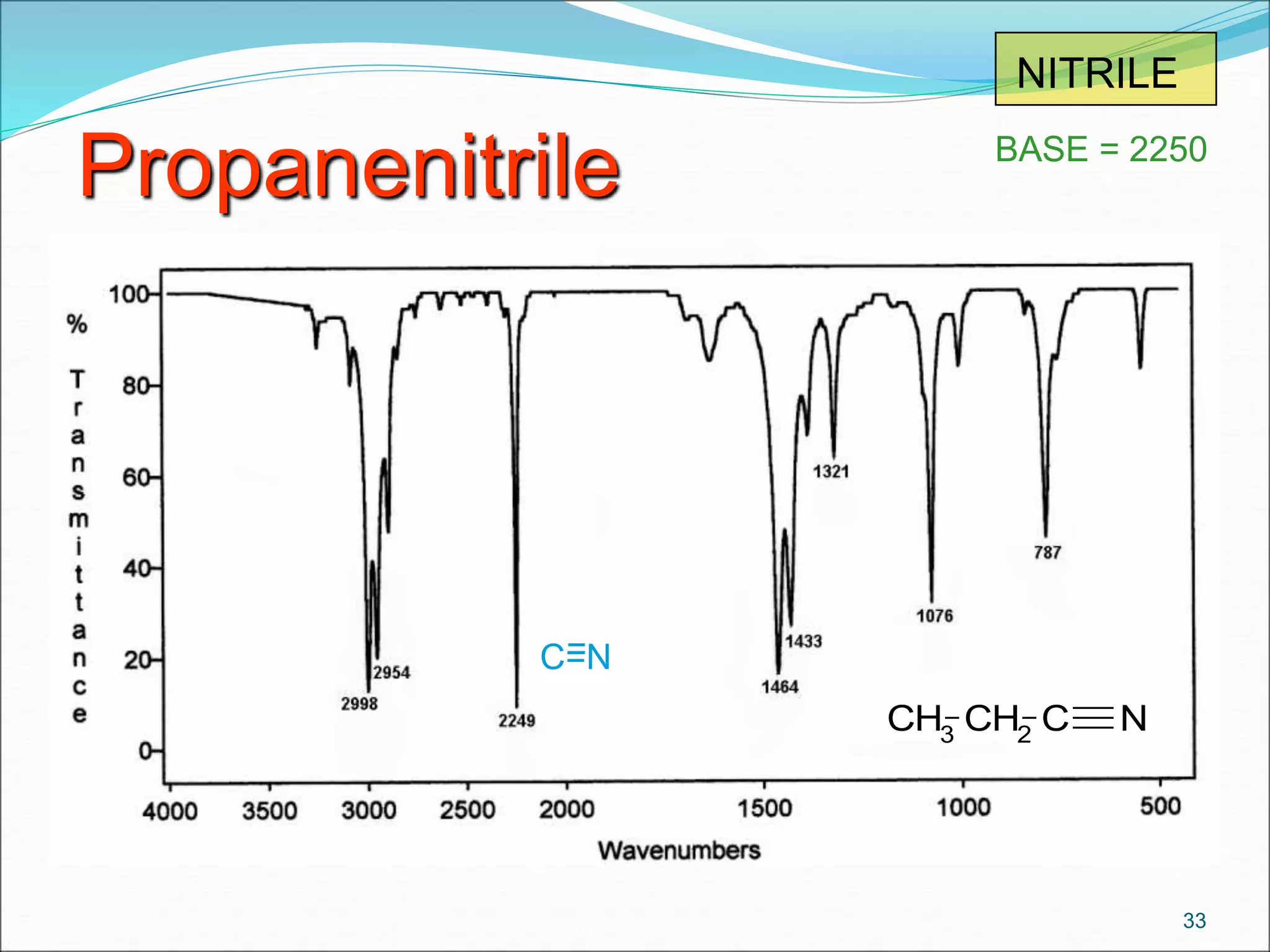
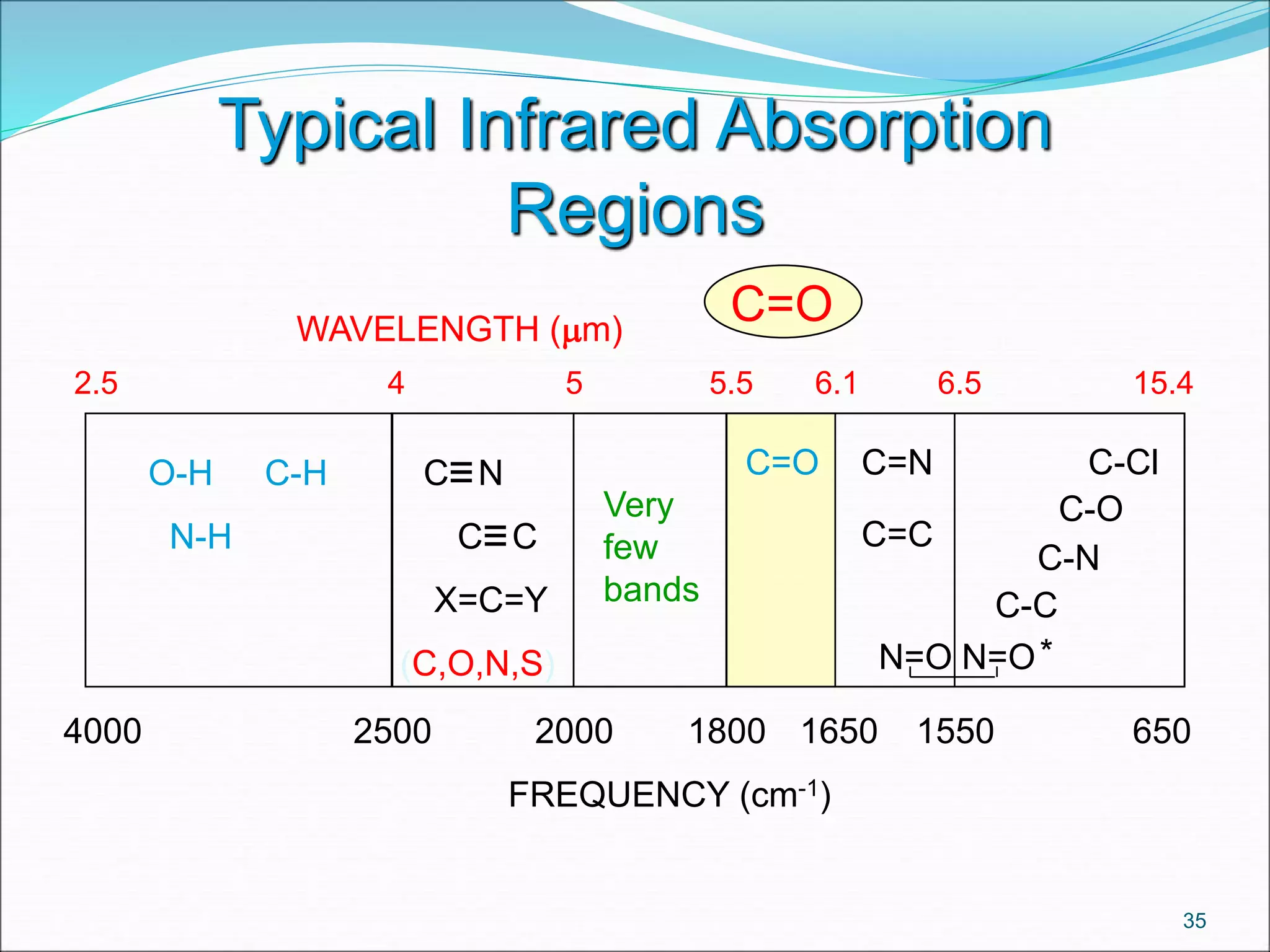
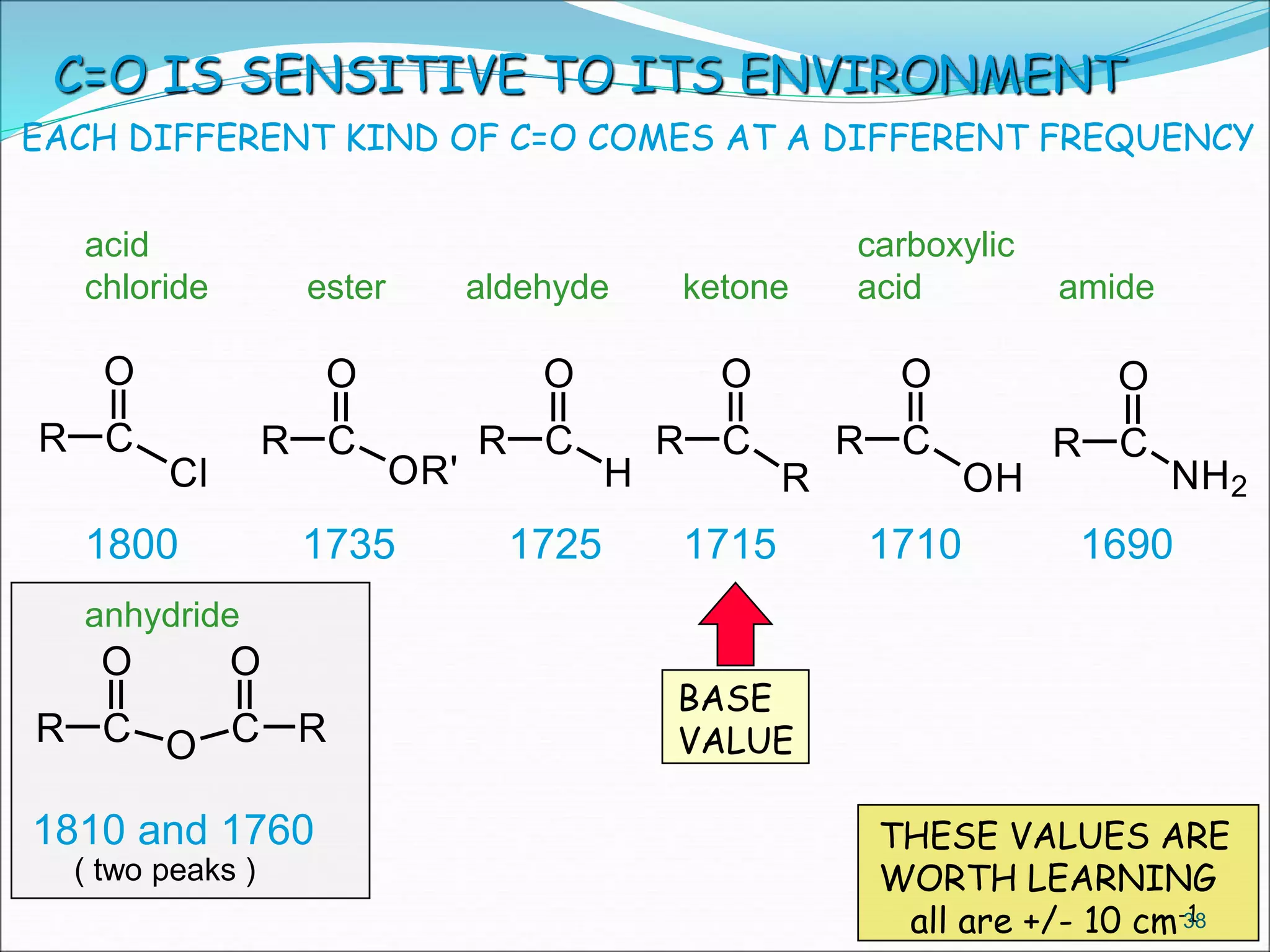
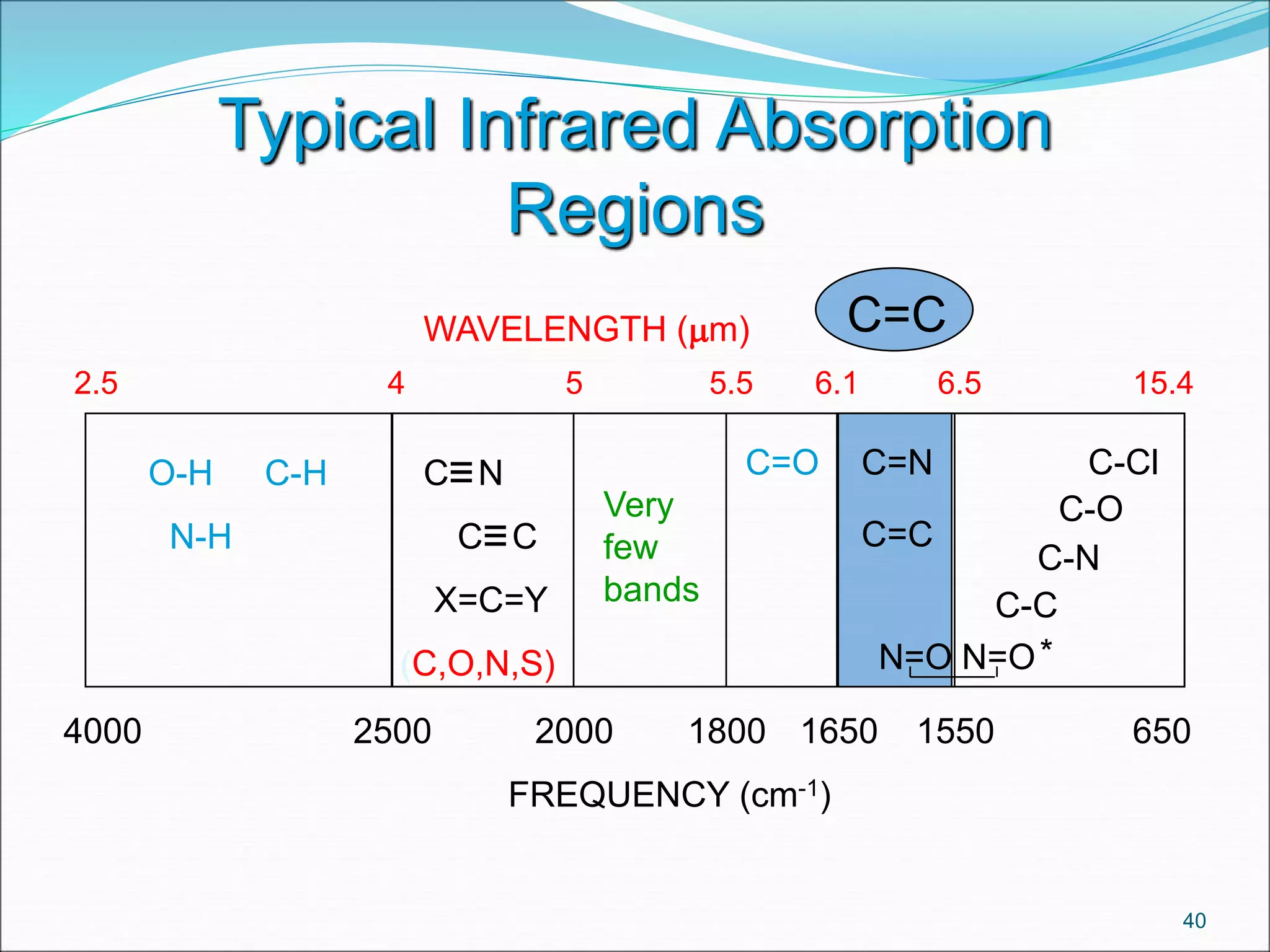
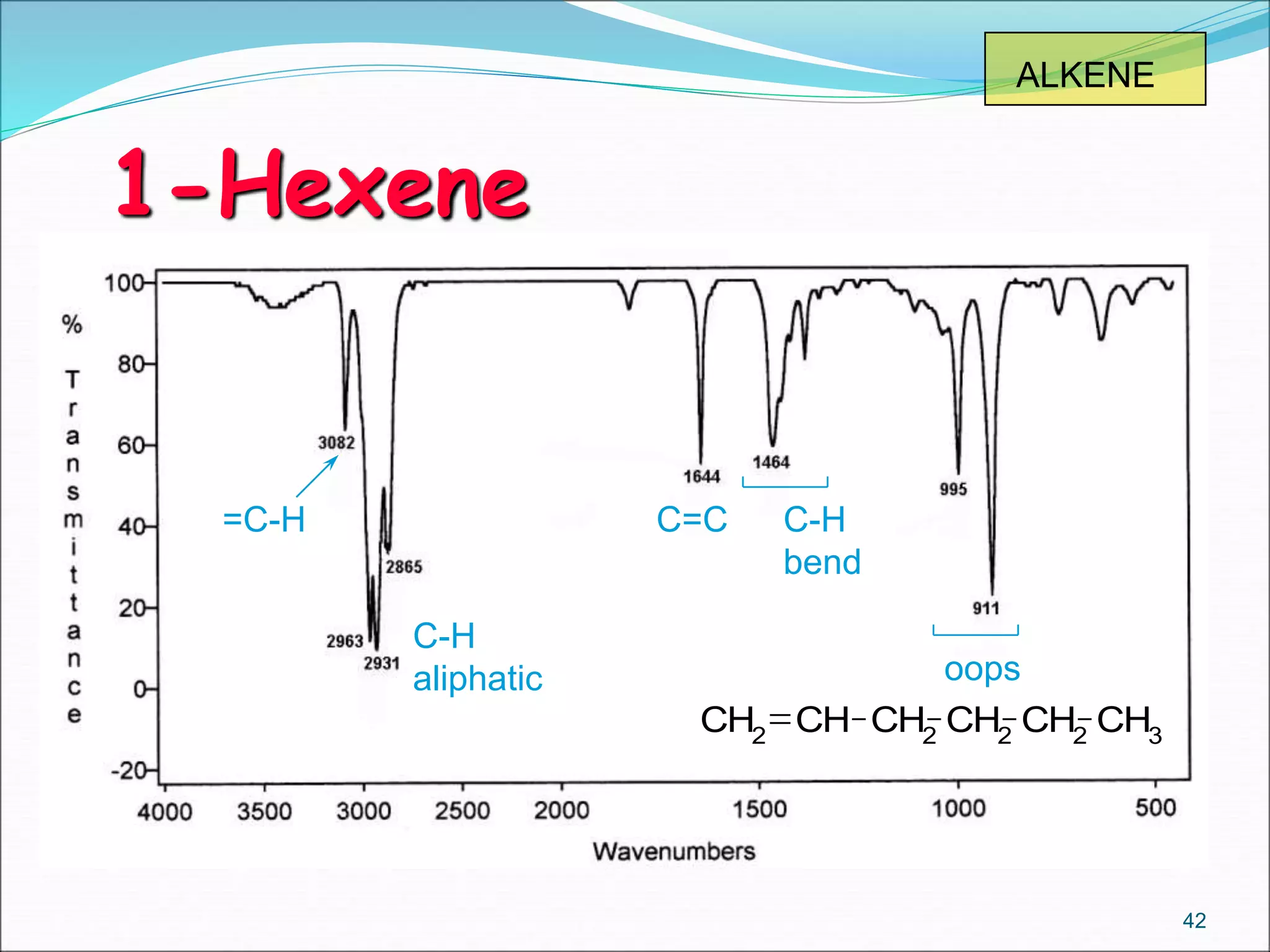
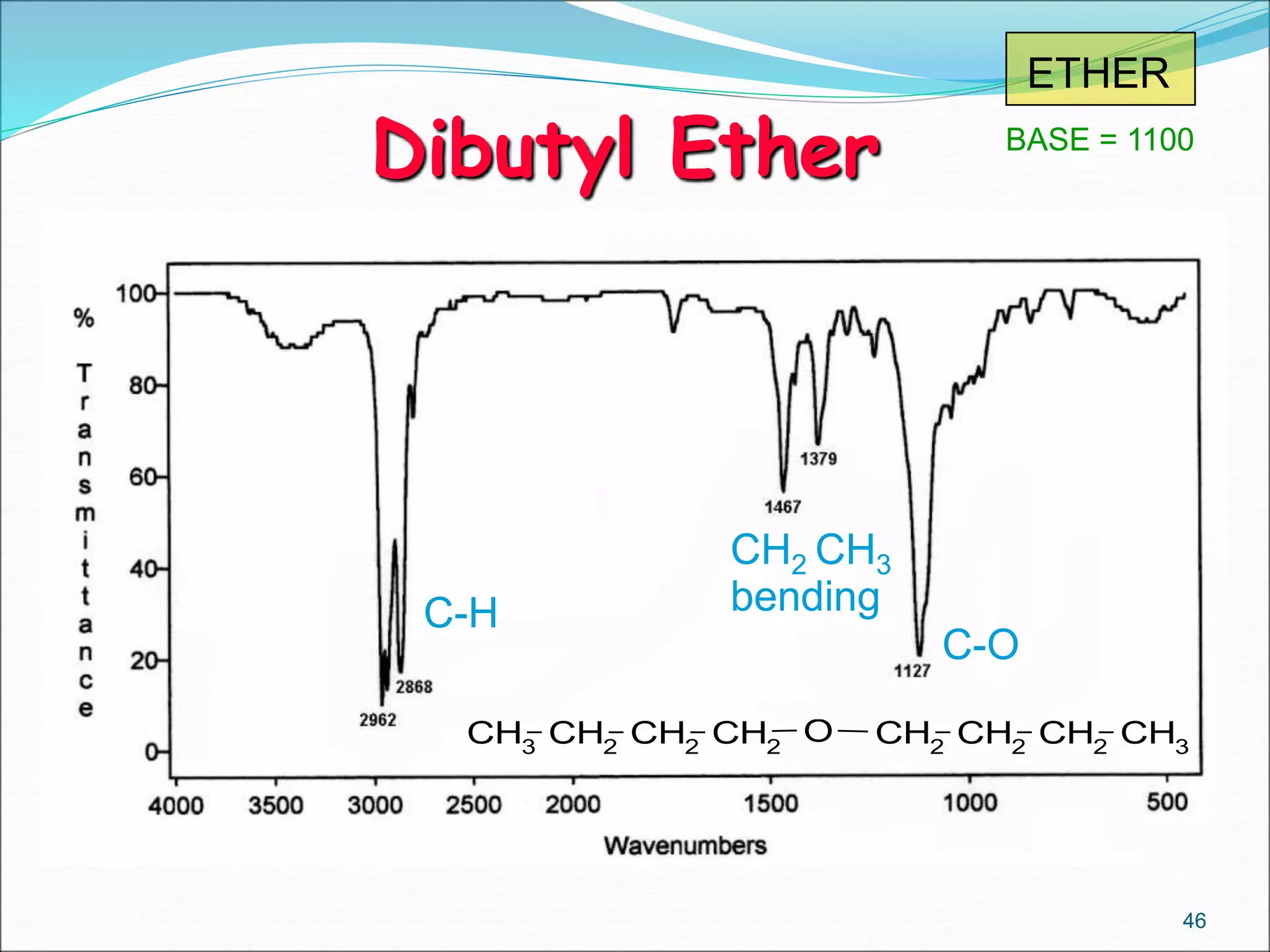
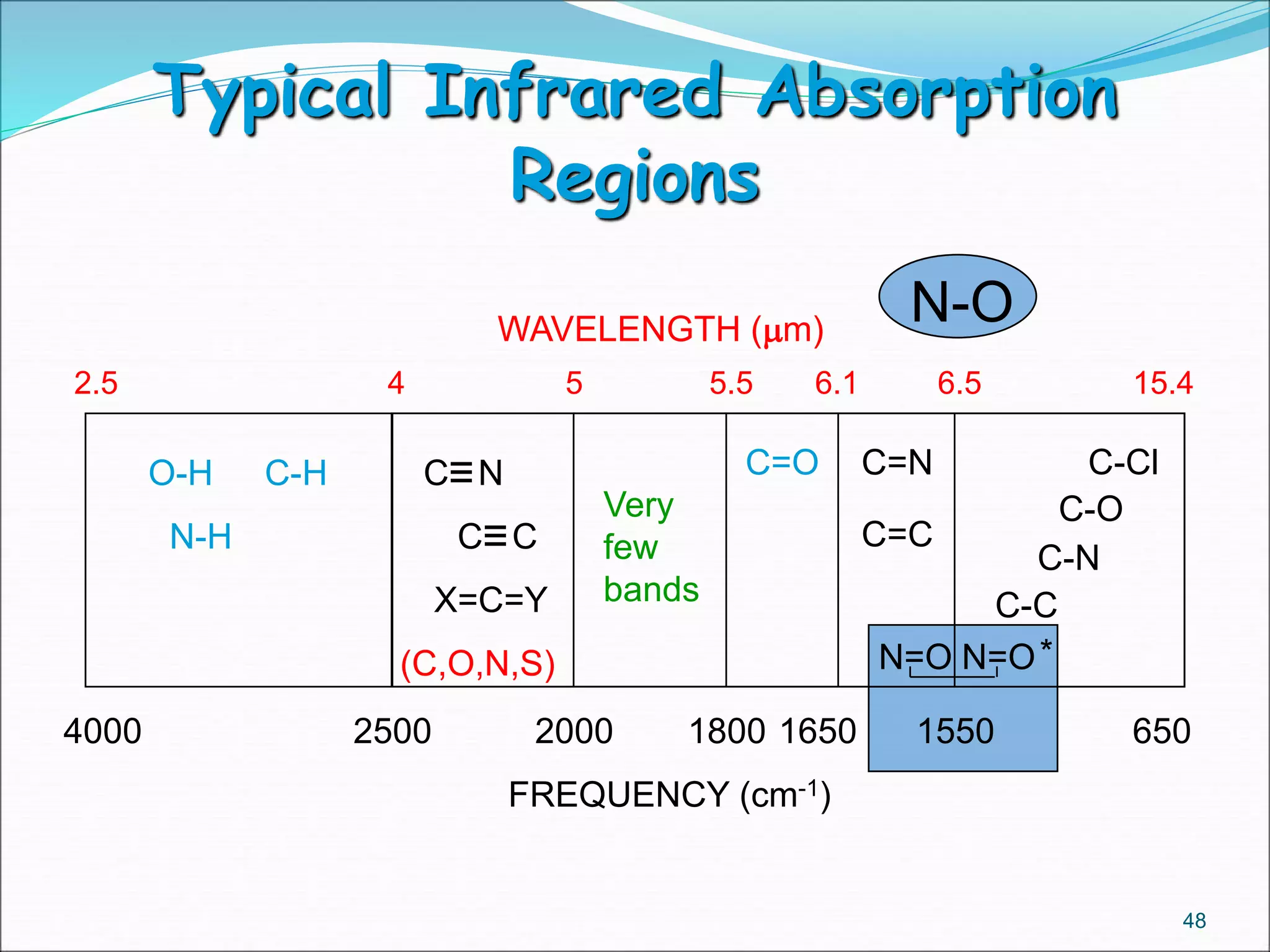
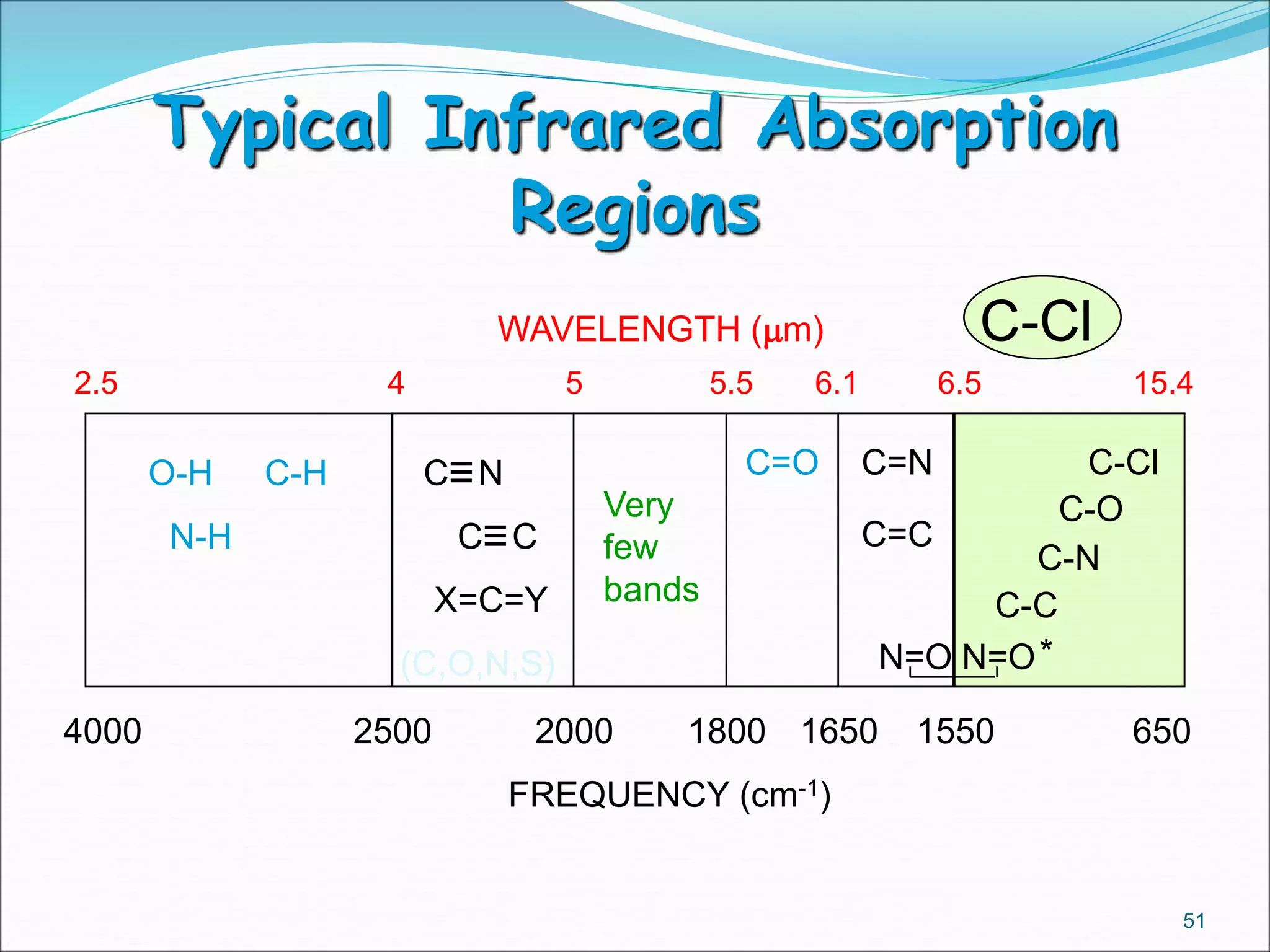
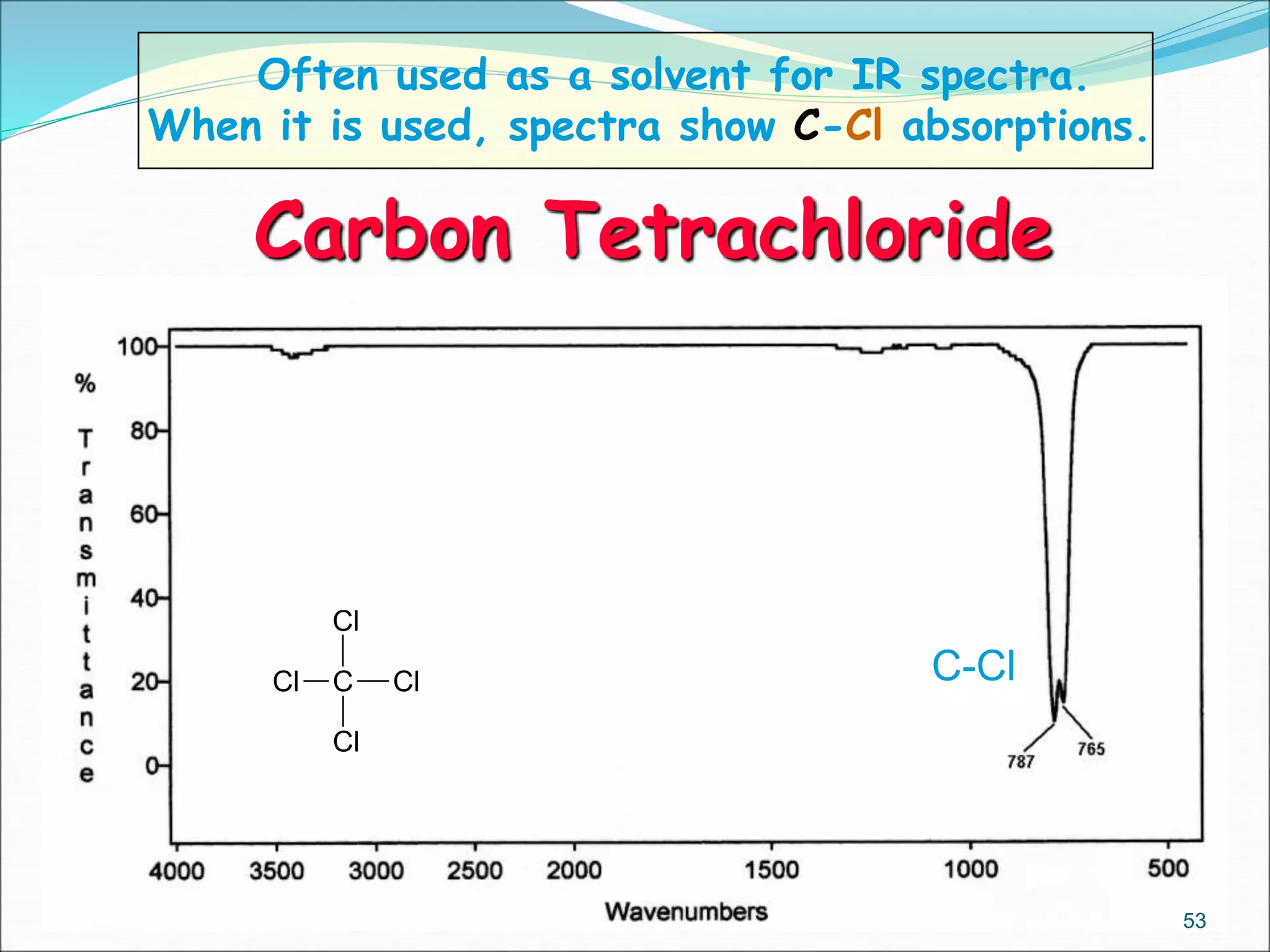
This document contains the slides from a seminar presentation on interpreting infrared spectroscopy. It begins with an overview of the principle and components of IR spectroscopy. It then discusses the different modes of molecular vibrations that can be observed in IR spectra, including stretching and bending vibrations. The document proceeds to explain the features of typical IR spectra and how they can be used. It concludes by interpreting various functional groups that can be identified in IR spectra, including O-H, N-H, C-H, C=O, C=C and others, based on their characteristic absorption regions.