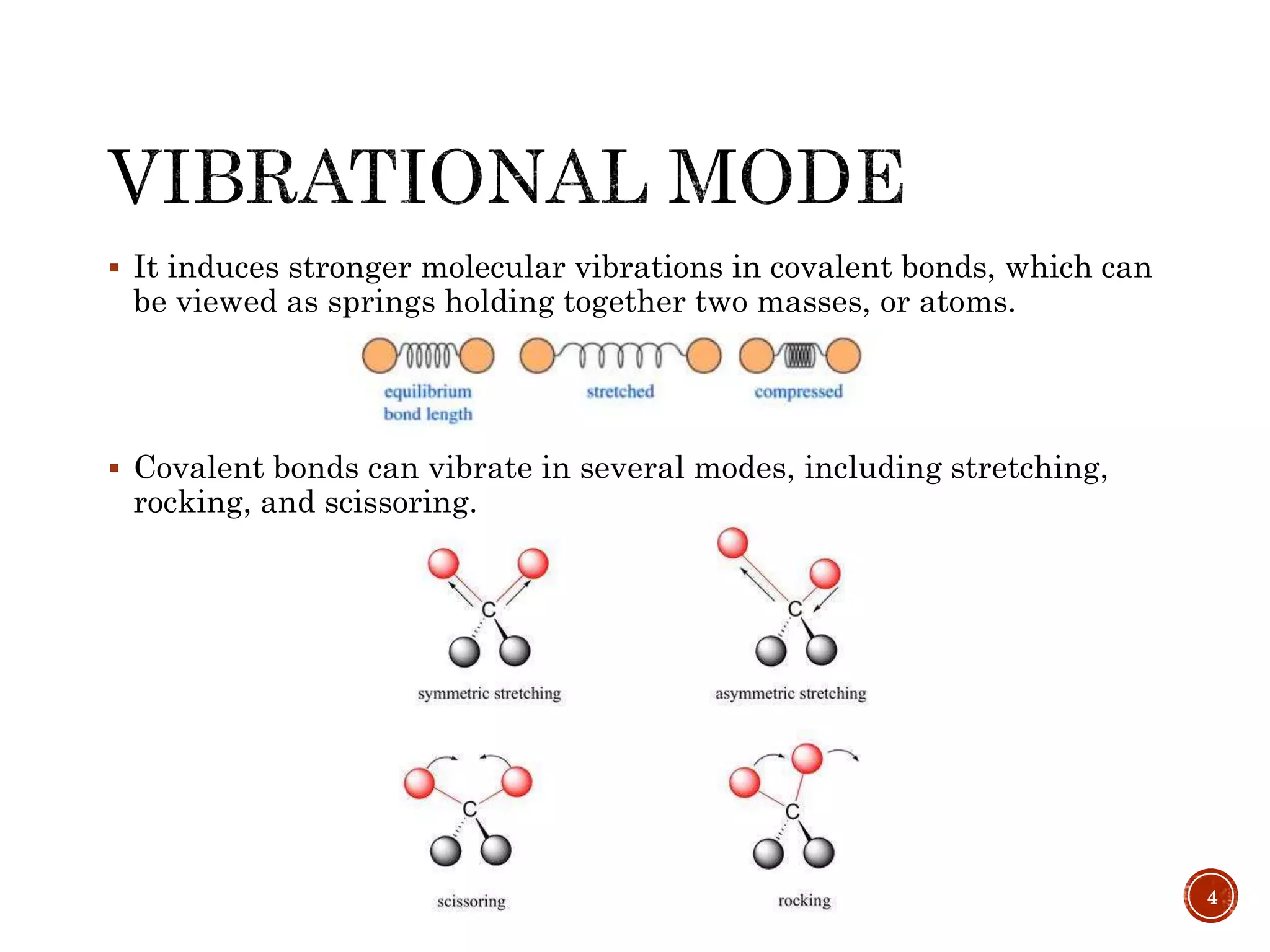
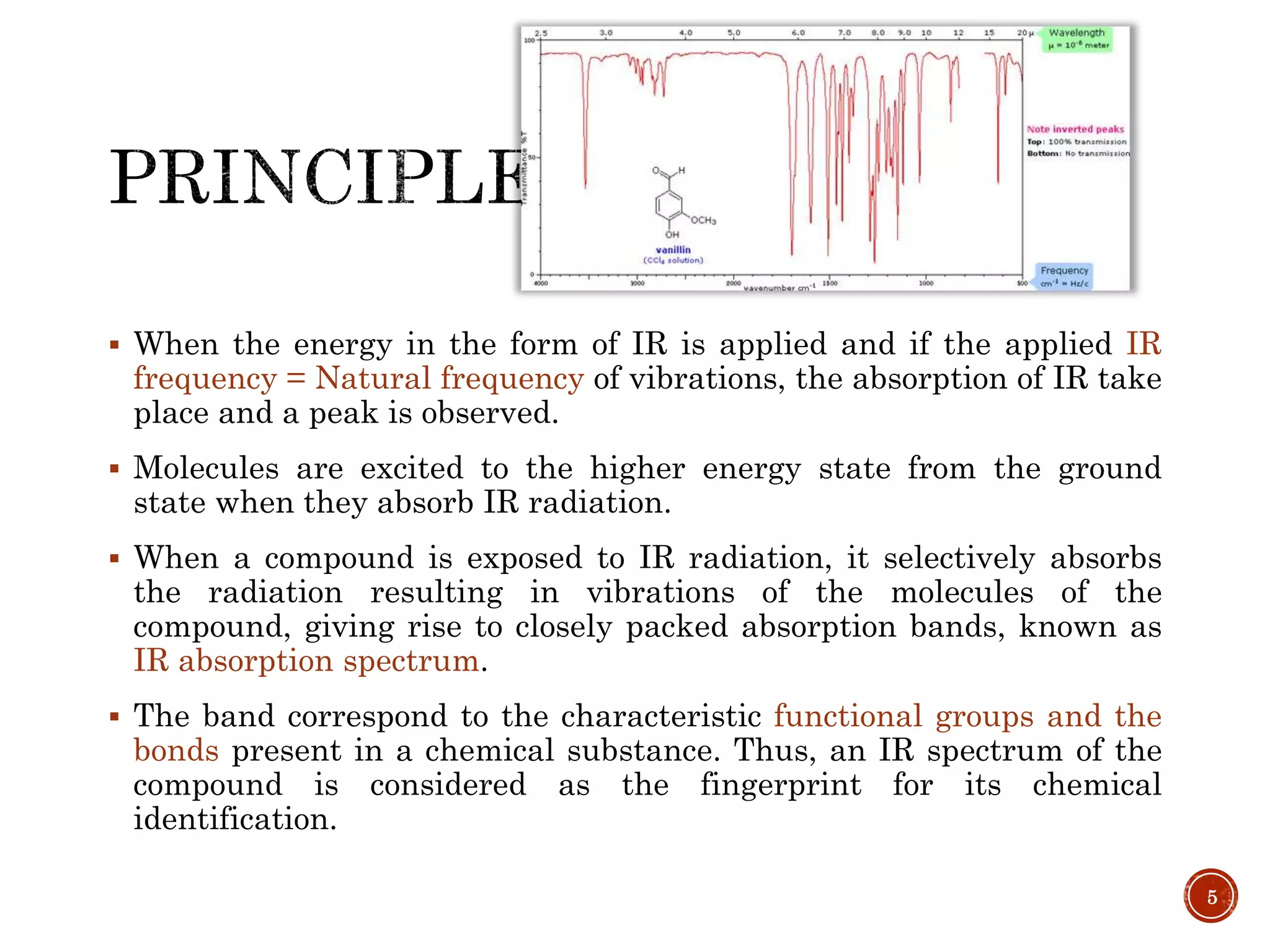
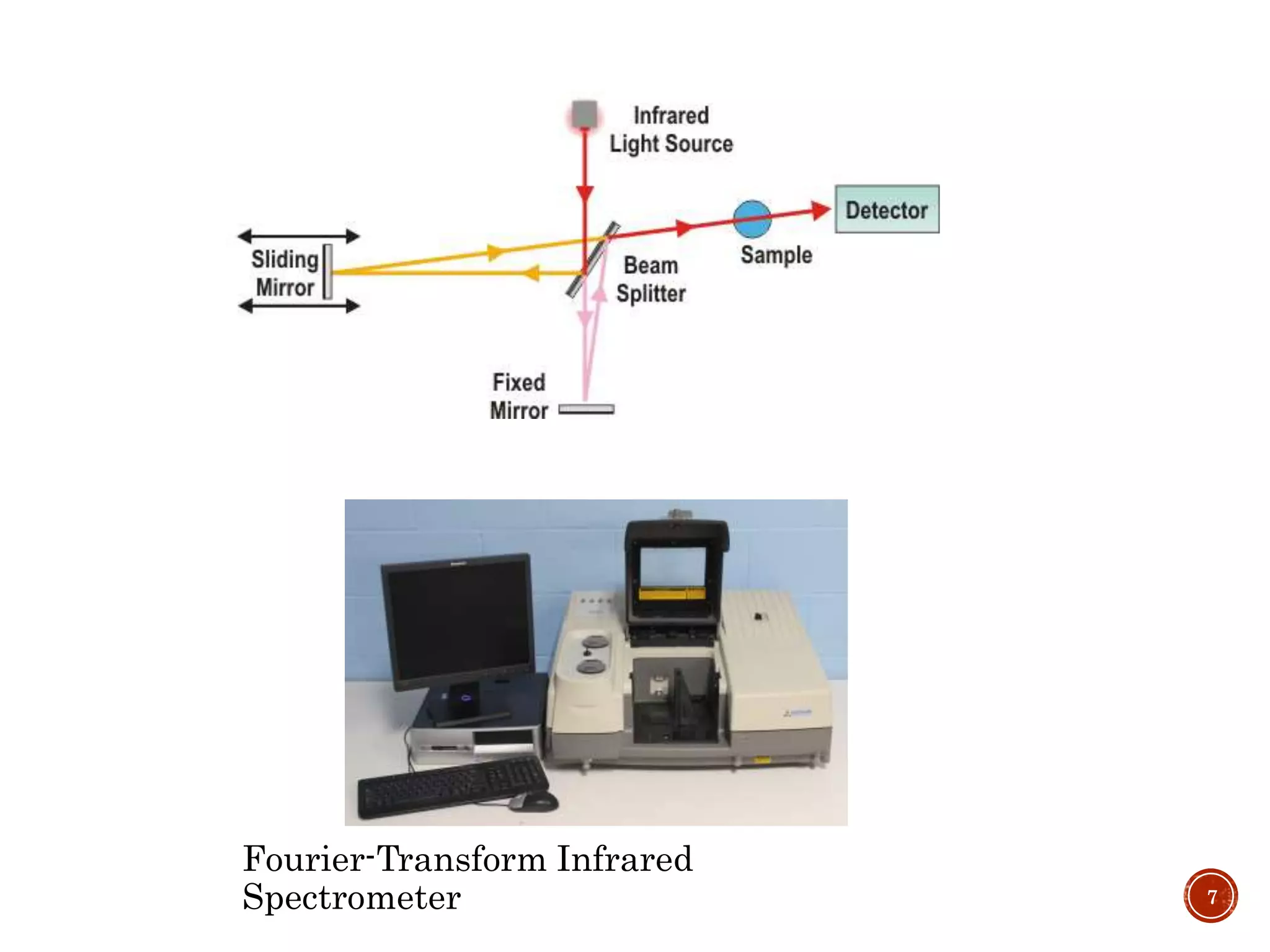
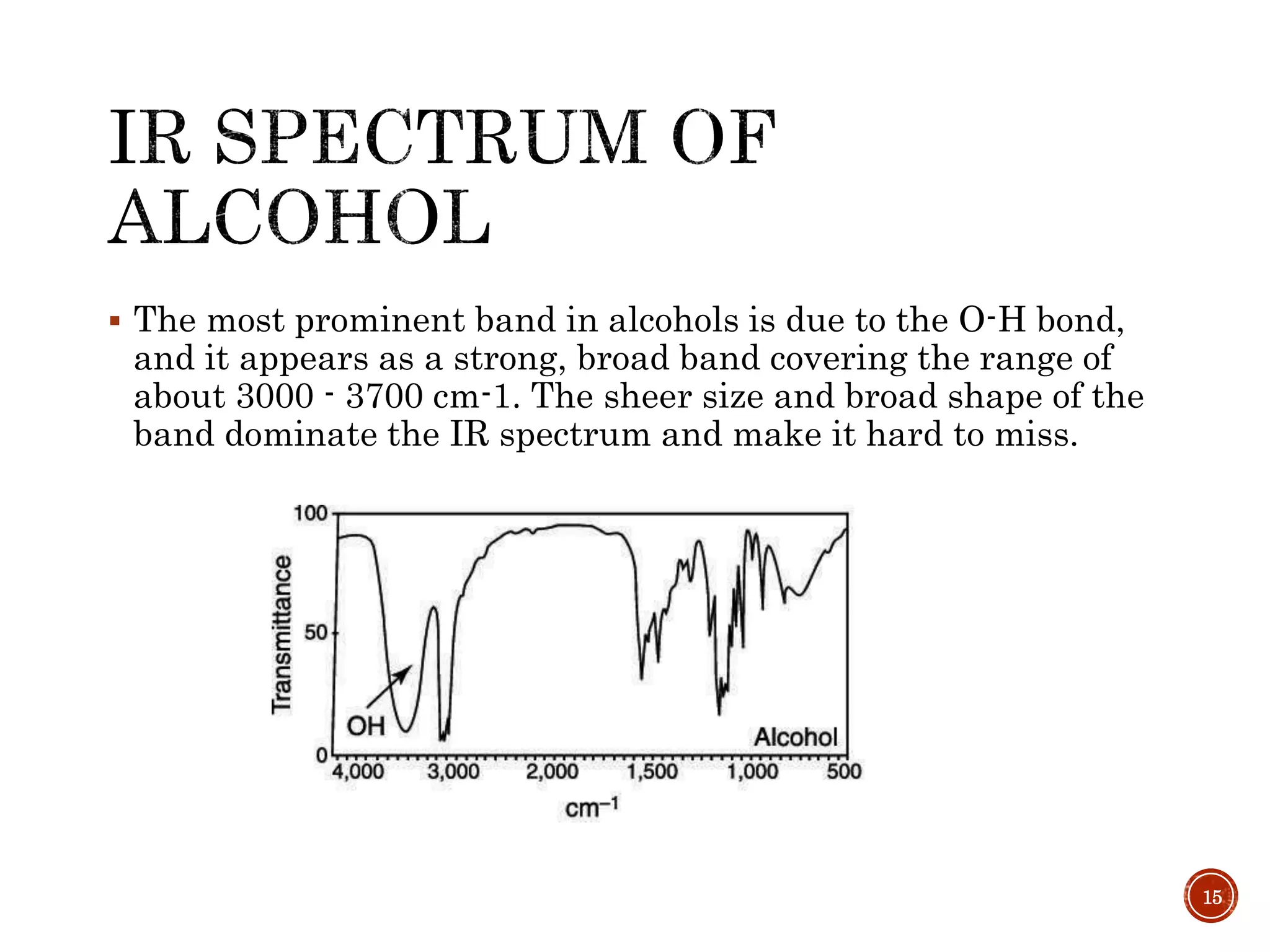
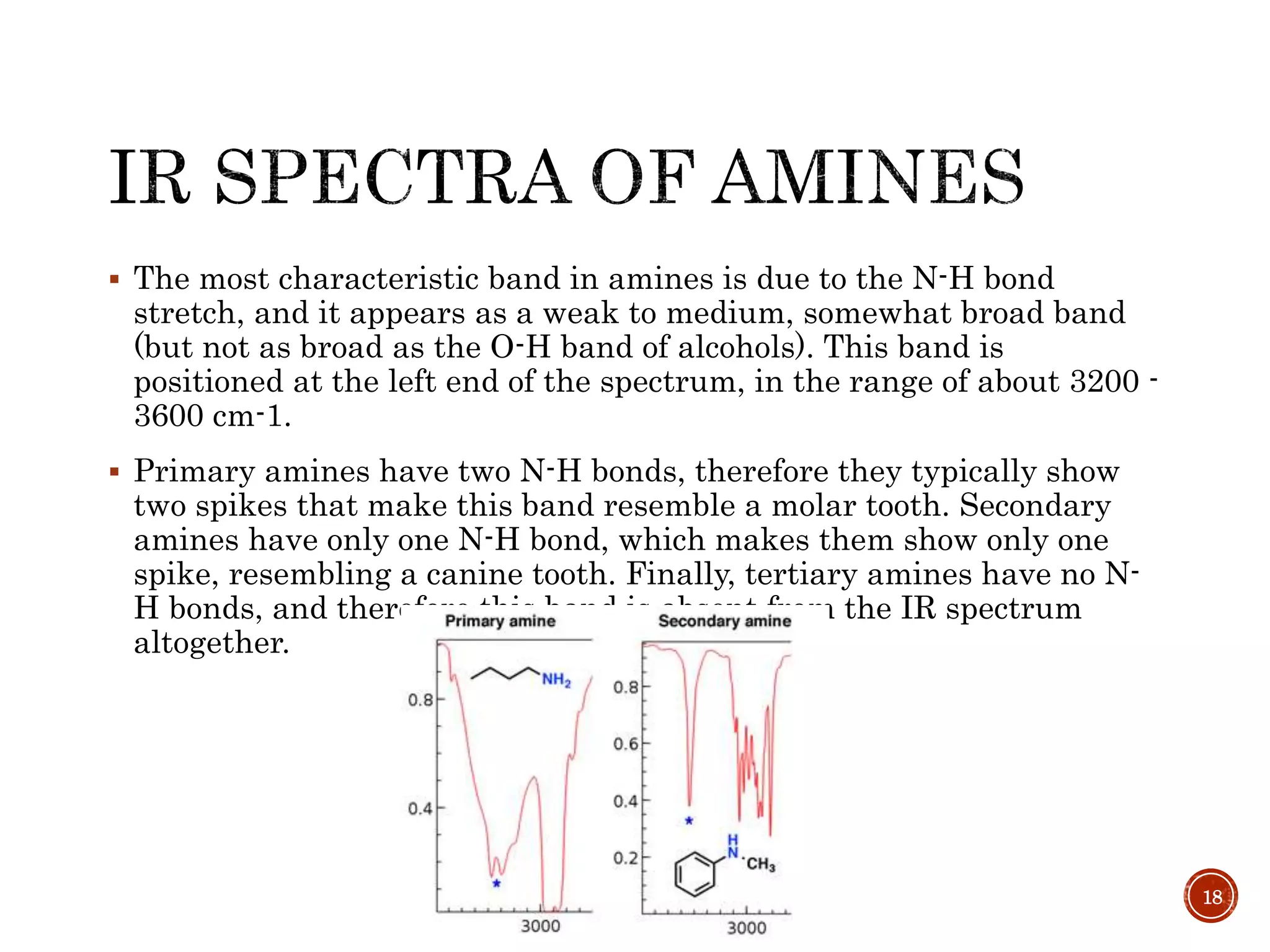
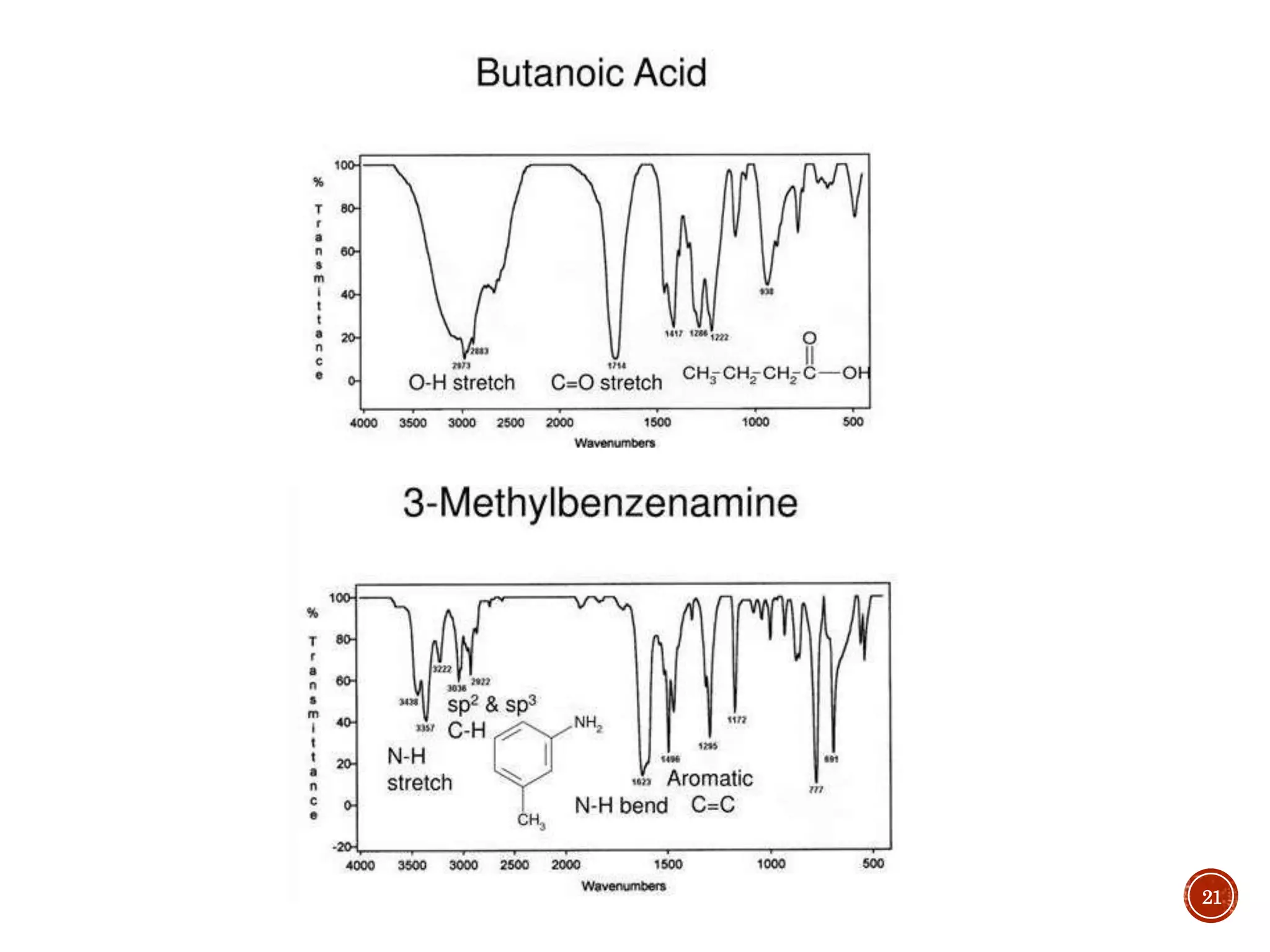
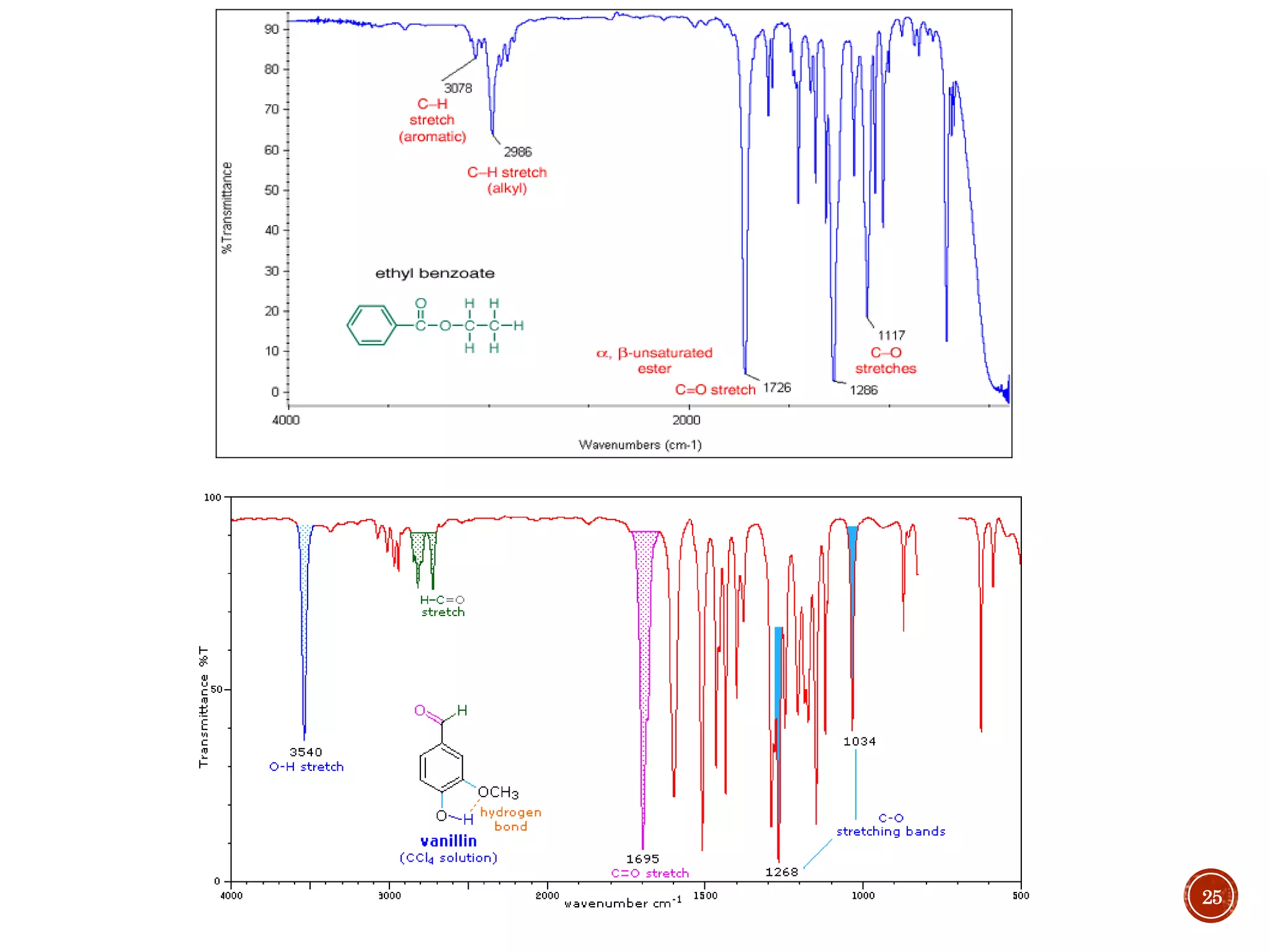
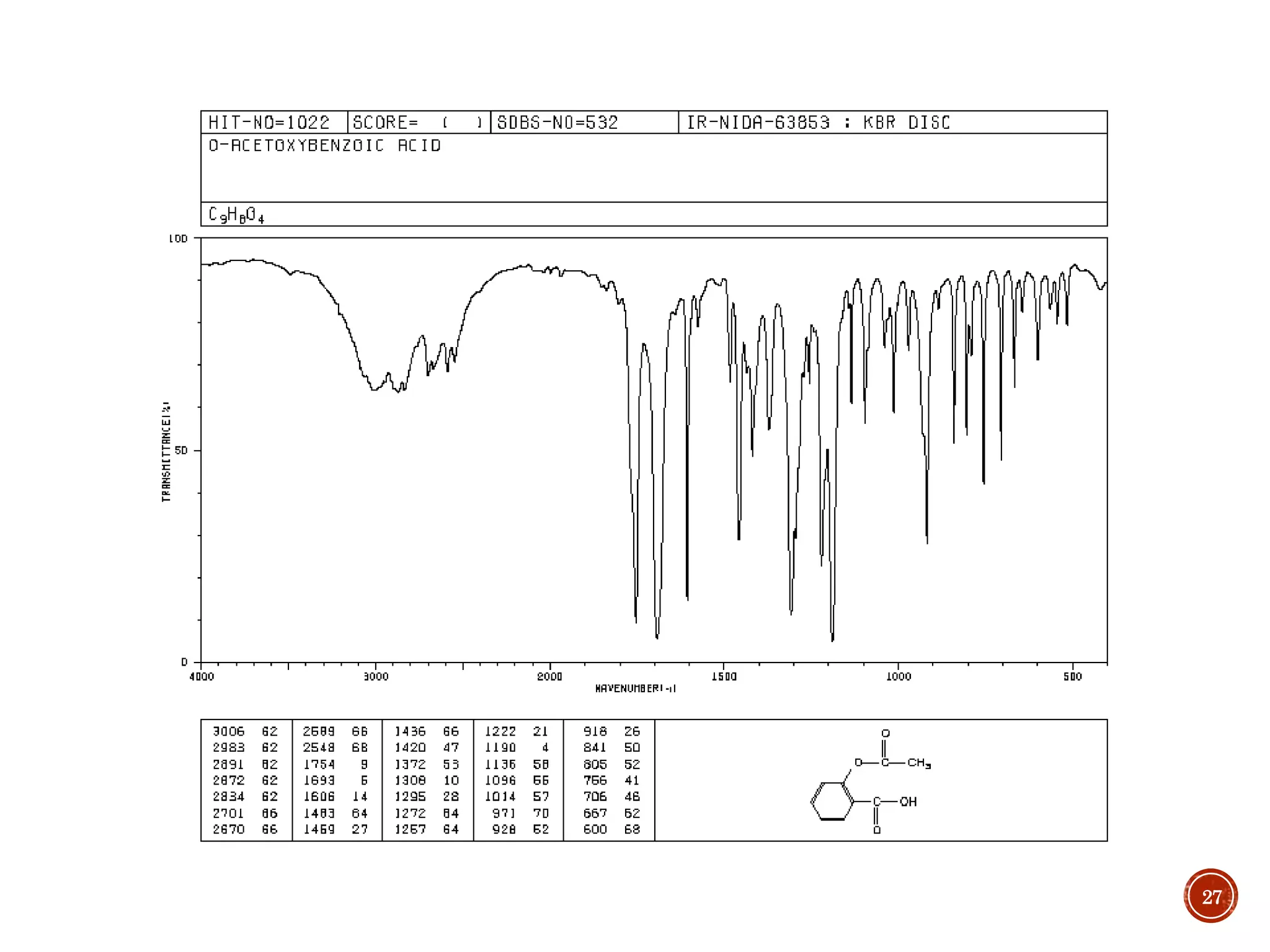
This document provides an overview of infrared (IR) spectroscopy. It discusses the basic principles of IR spectroscopy, including that IR radiation induces molecular vibrations that can be measured to determine a molecule's structure. It describes the typical instrumentation used, including sources of IR radiation and detectors. It explains how different functional groups absorb IR radiation at characteristic frequencies. Examples are provided of the IR spectra of common organic functional groups like alkenes, alkynes, alcohols, and amines. Applications of IR spectroscopy in fields like pharmaceutical analysis and polymer characterization are mentioned. Advantages include speed and applicability to many sample types, while disadvantages include some sample destruction and inability to analyze non-IR-active compounds.