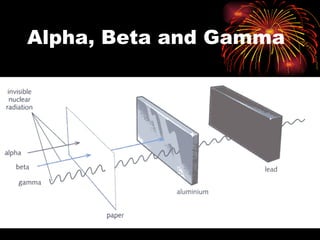
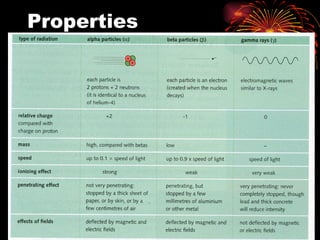
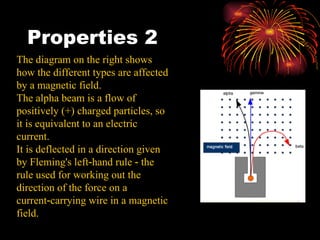
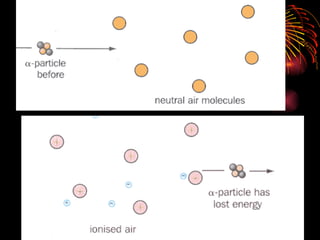
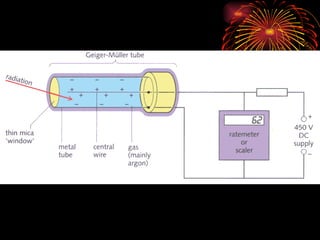
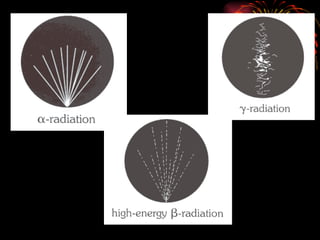
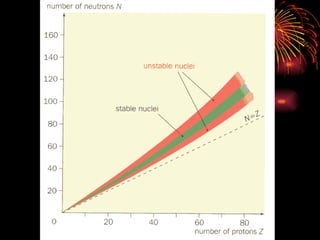
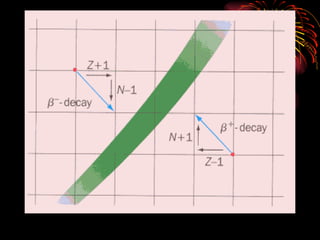
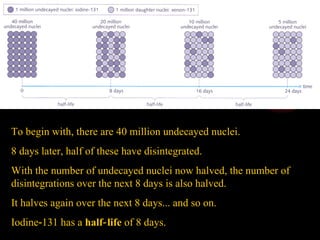
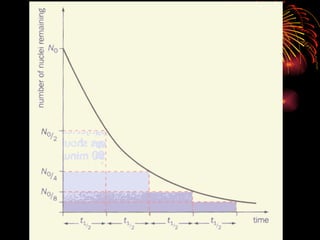
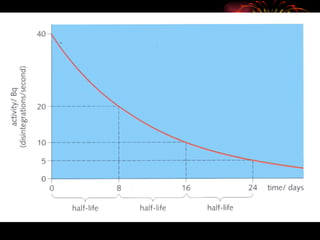
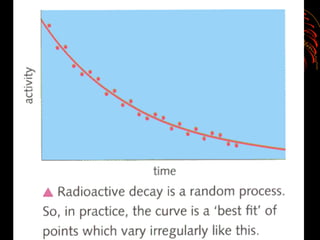
The document discusses different types of radioactive decay and radiation. It describes alpha, beta, and gamma radiation, and how they interact with and penetrate matter differently due to their mass, charge, and energy. Alpha particles interact strongly, penetrate only a short distance, and produce many ion pairs. Beta particles penetrate farther than alphas as they are lighter and slower. Gamma rays interact weakly and penetrate the deepest as they are uncharged photons. The document also defines half-life as the time for half the nuclei in a sample or half the sample's activity to decay.