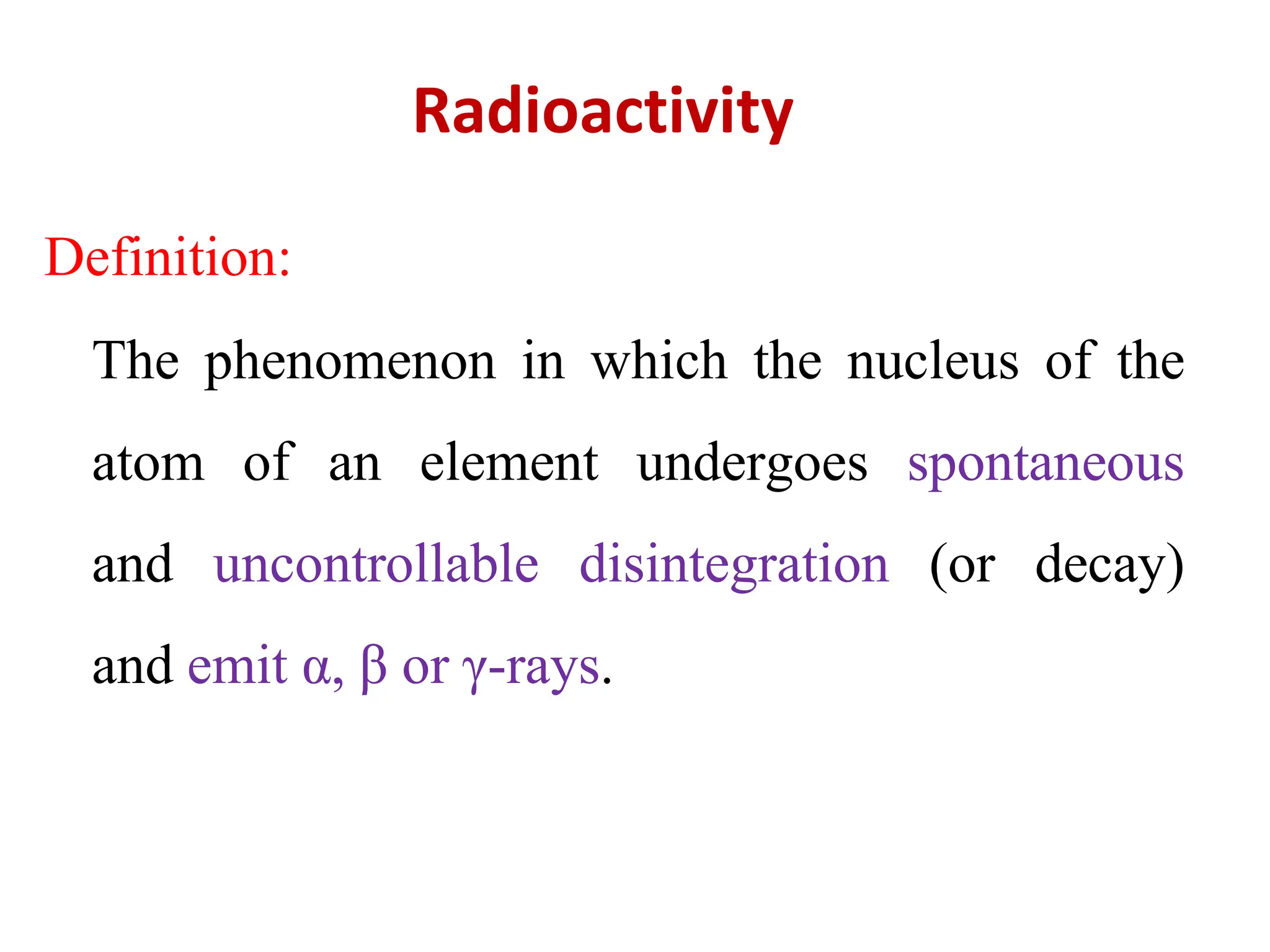
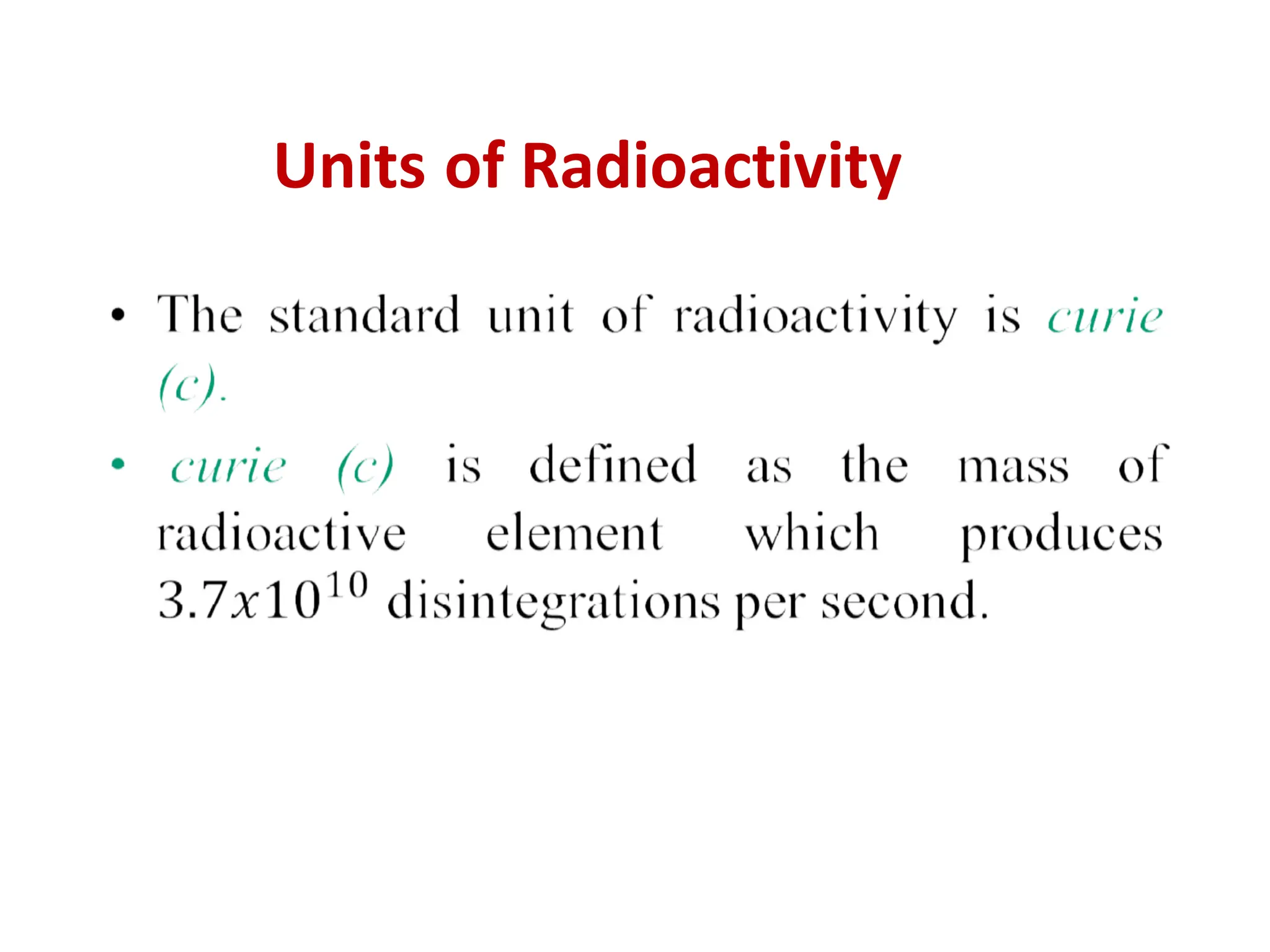
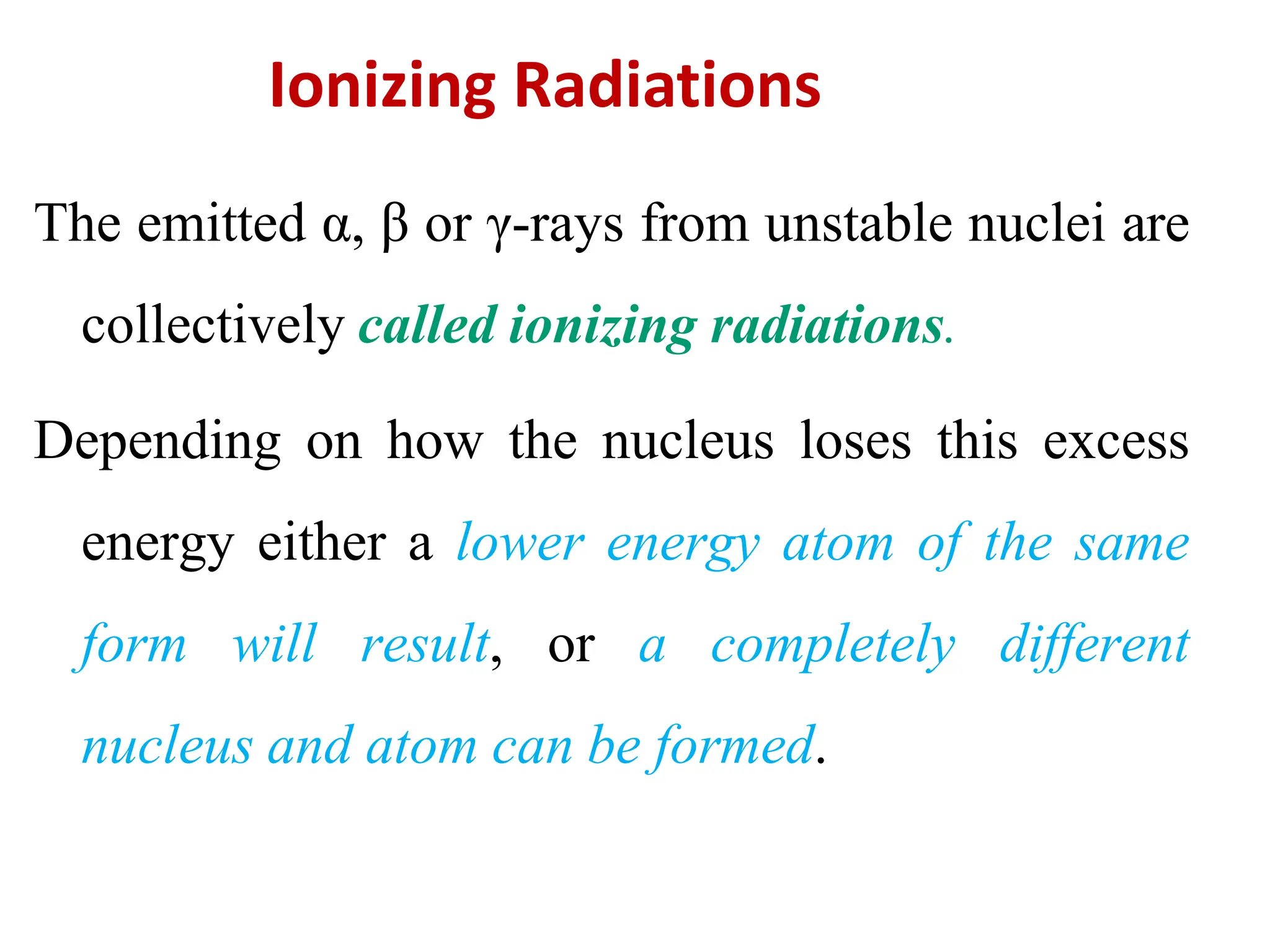
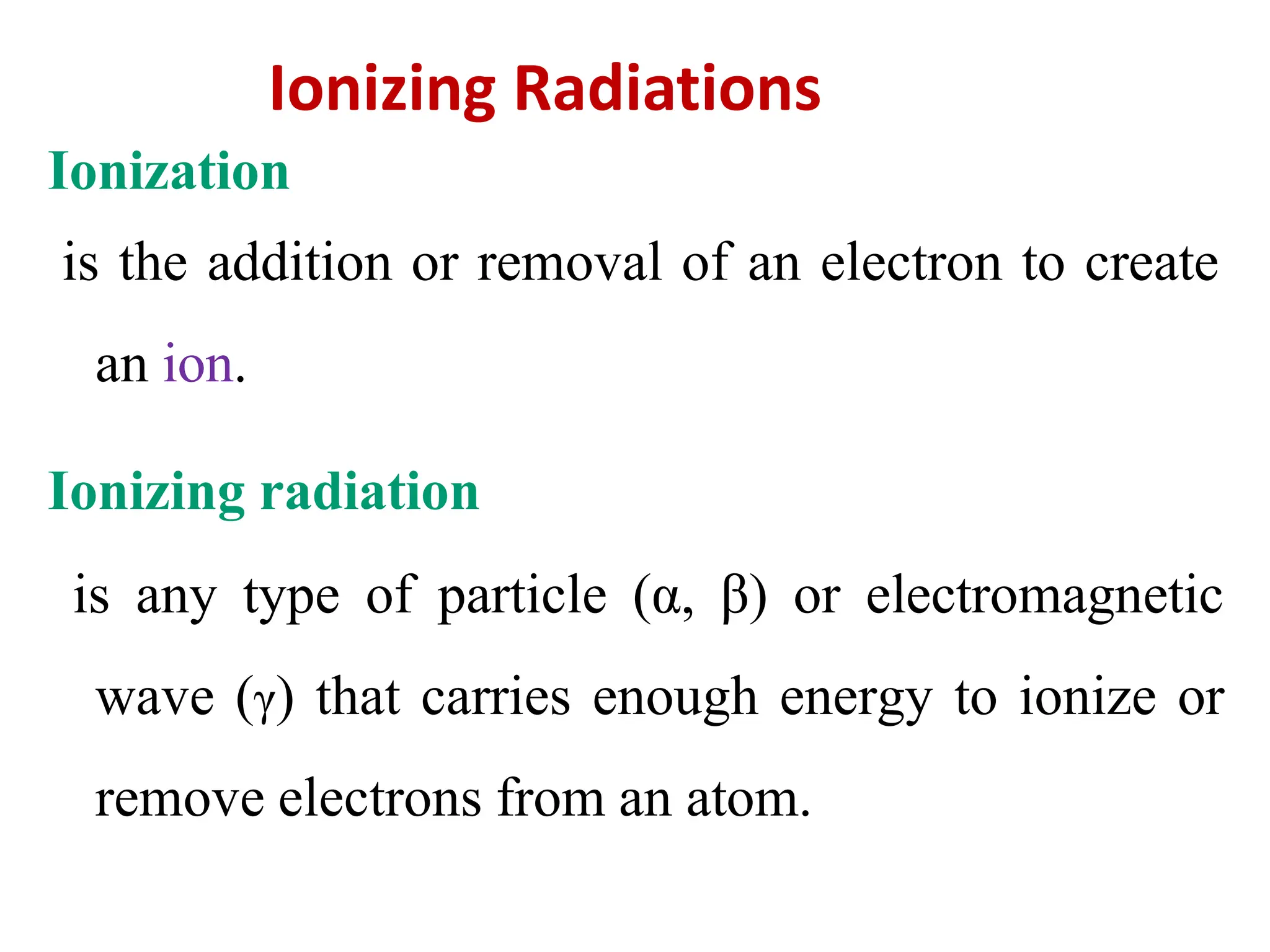
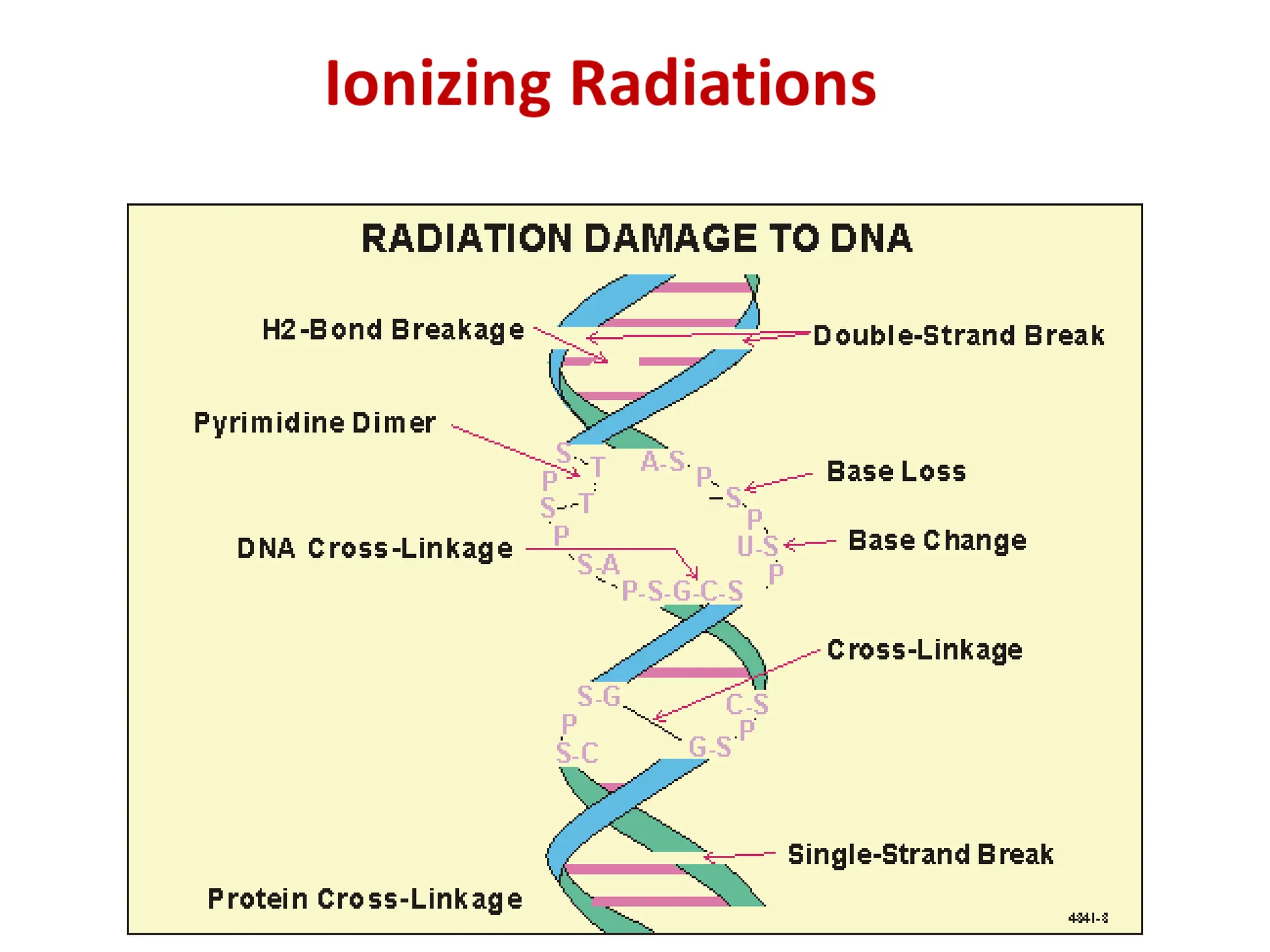
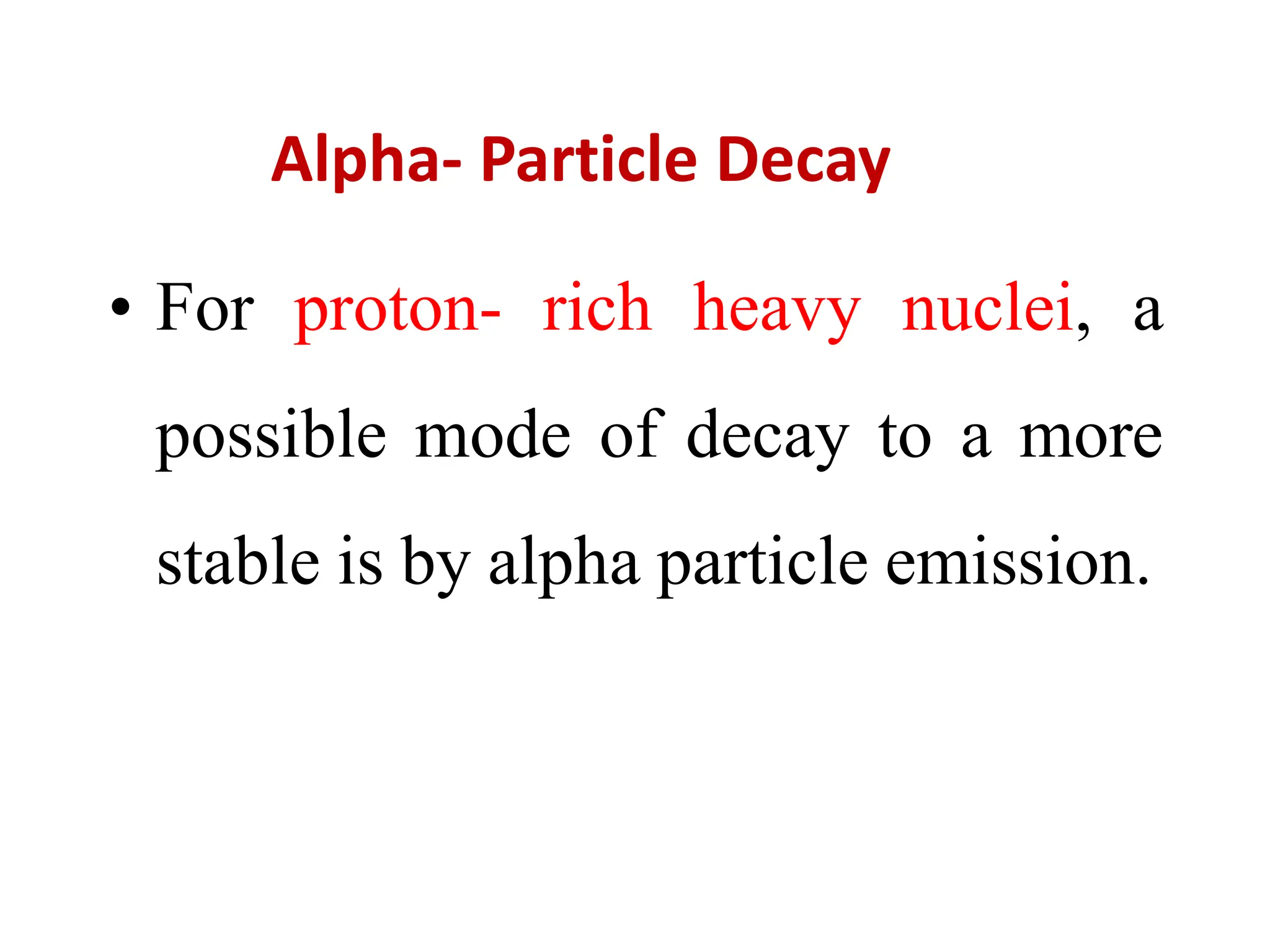
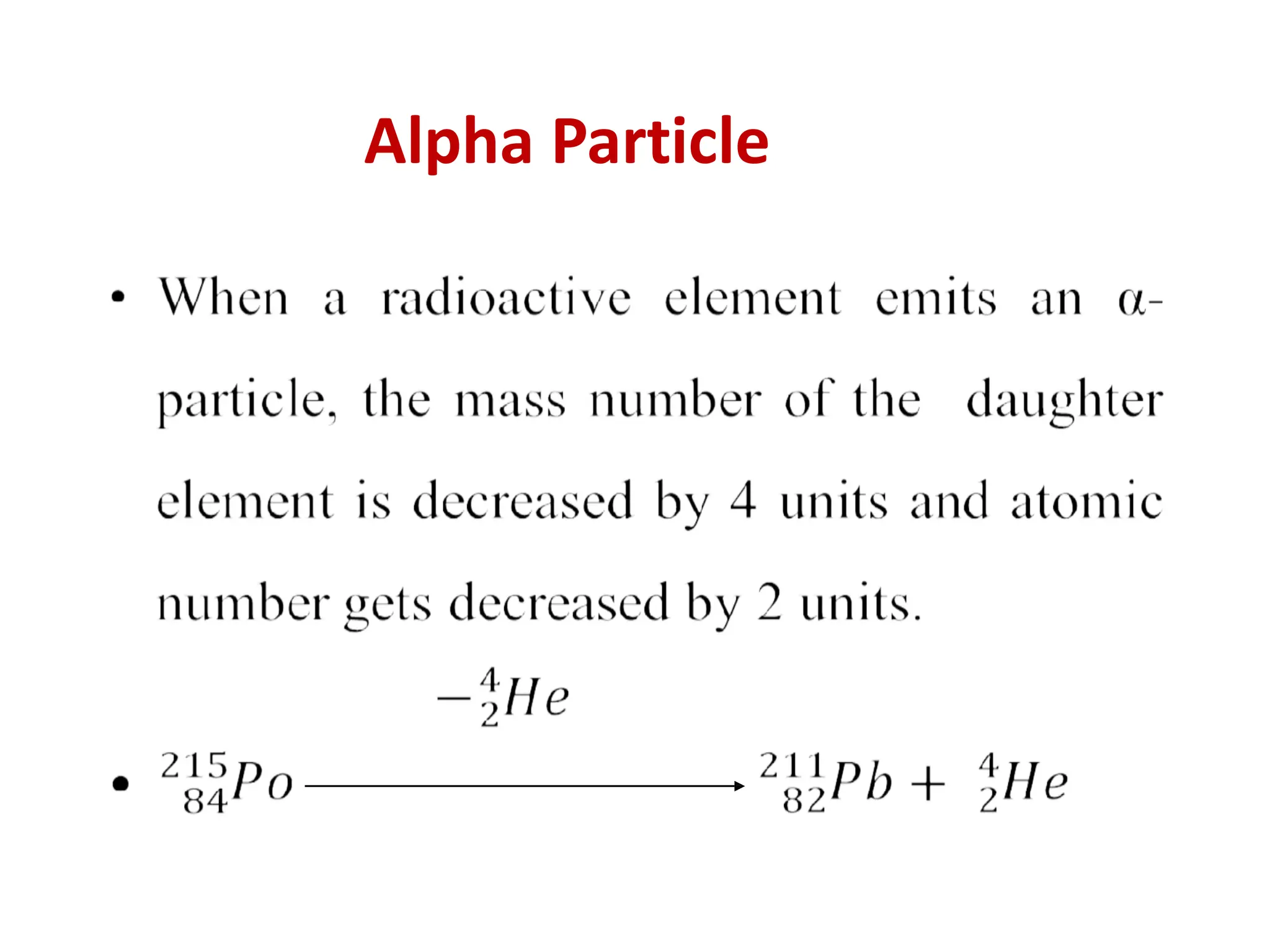
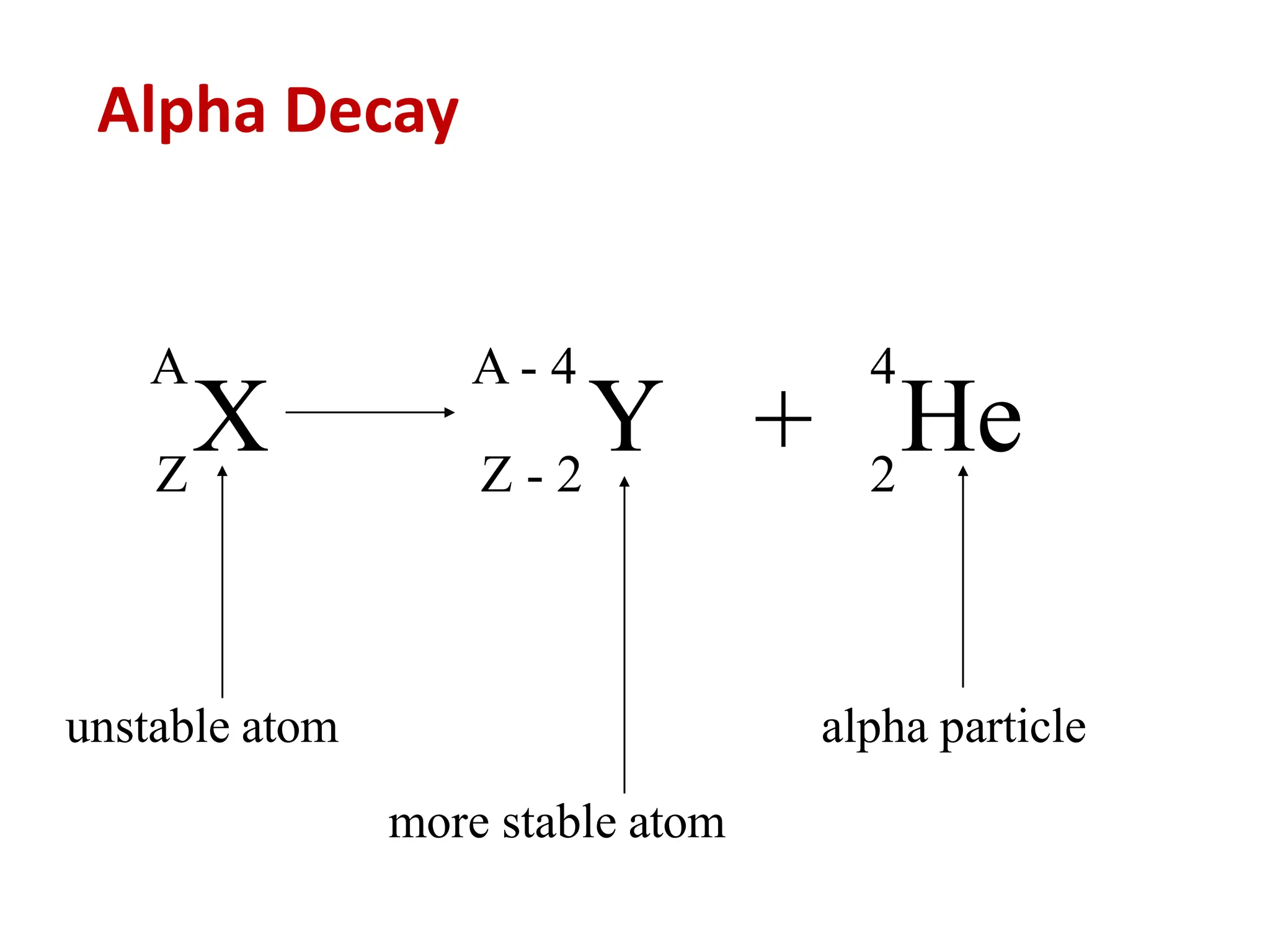
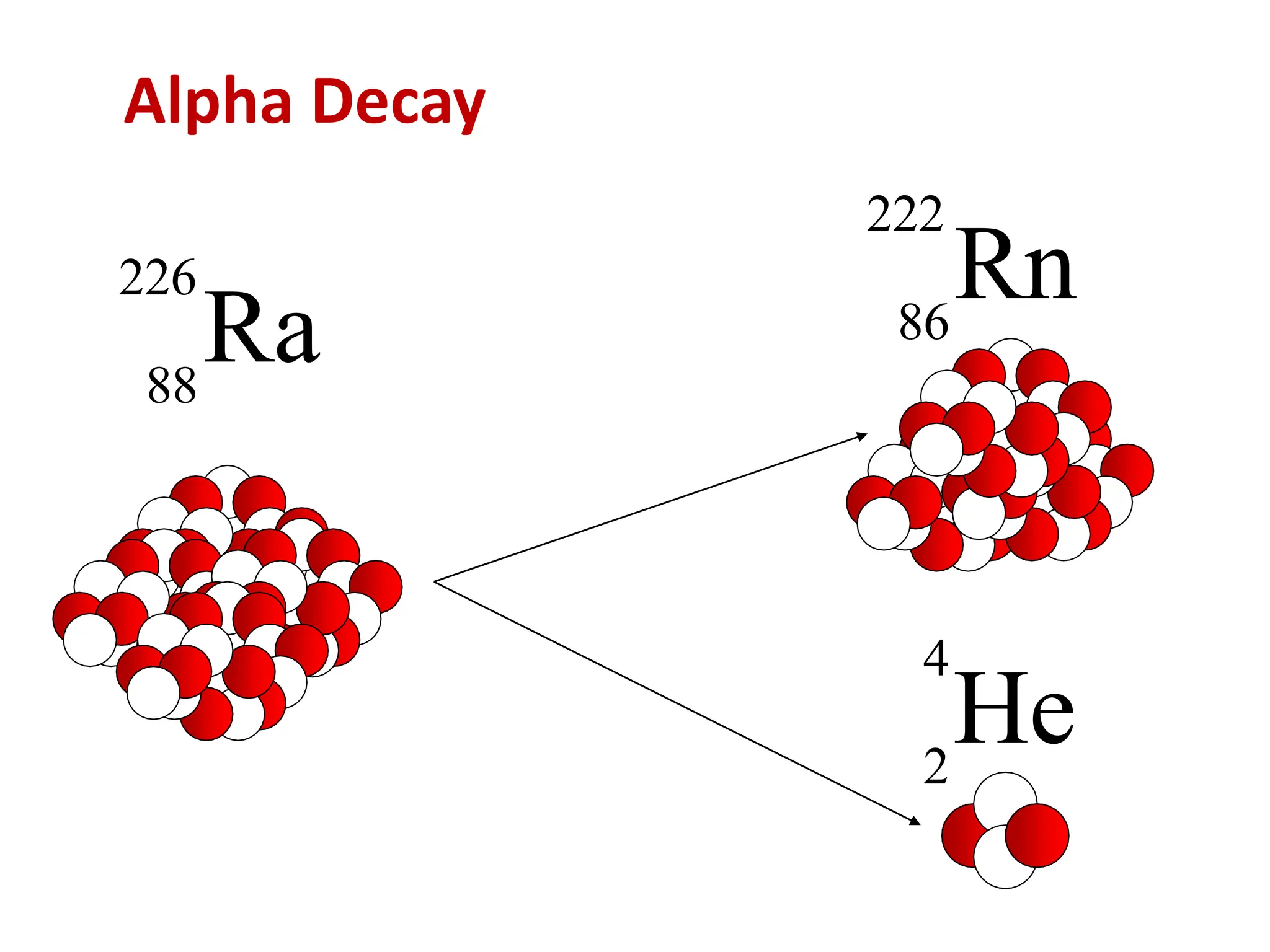
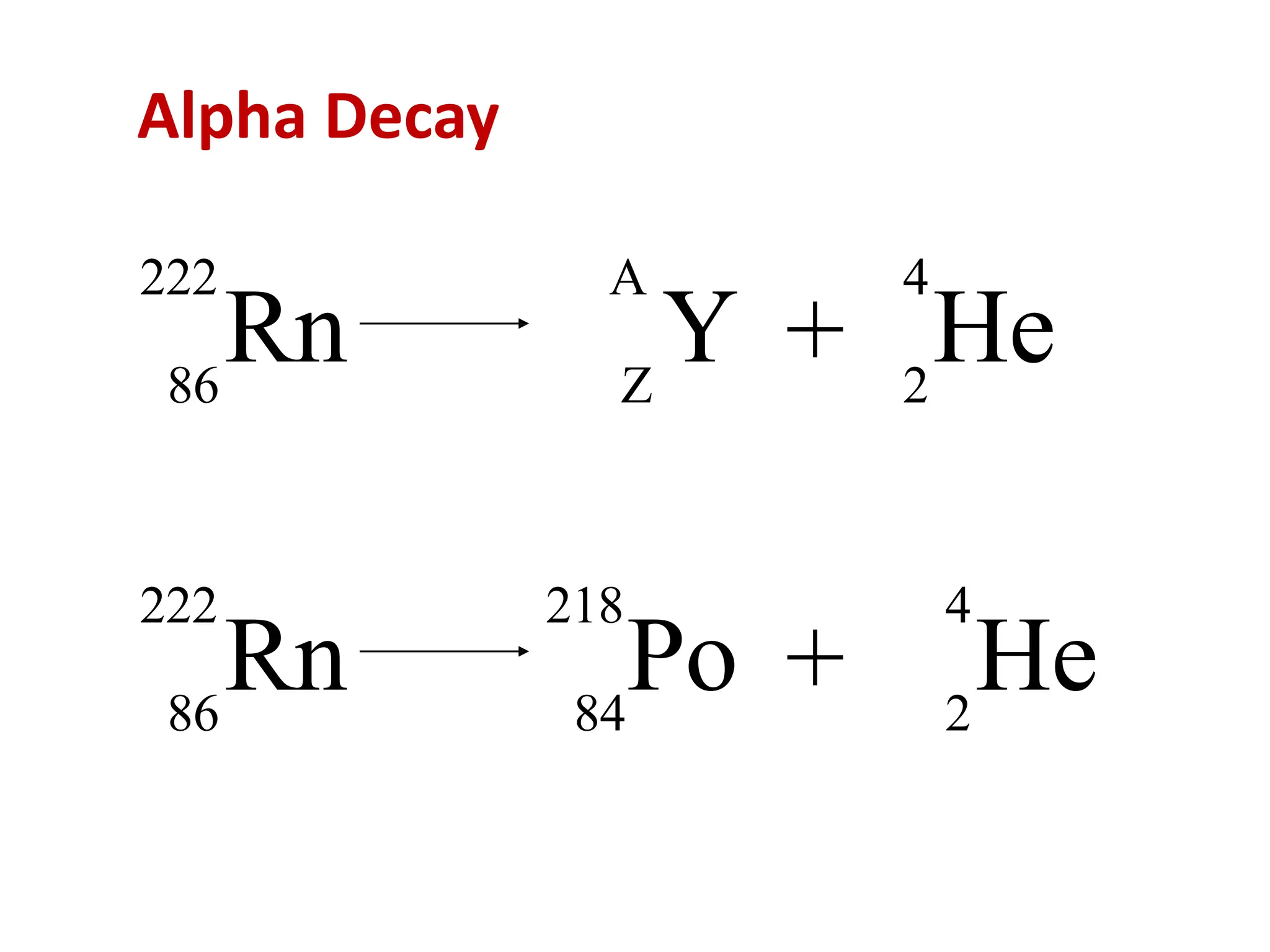
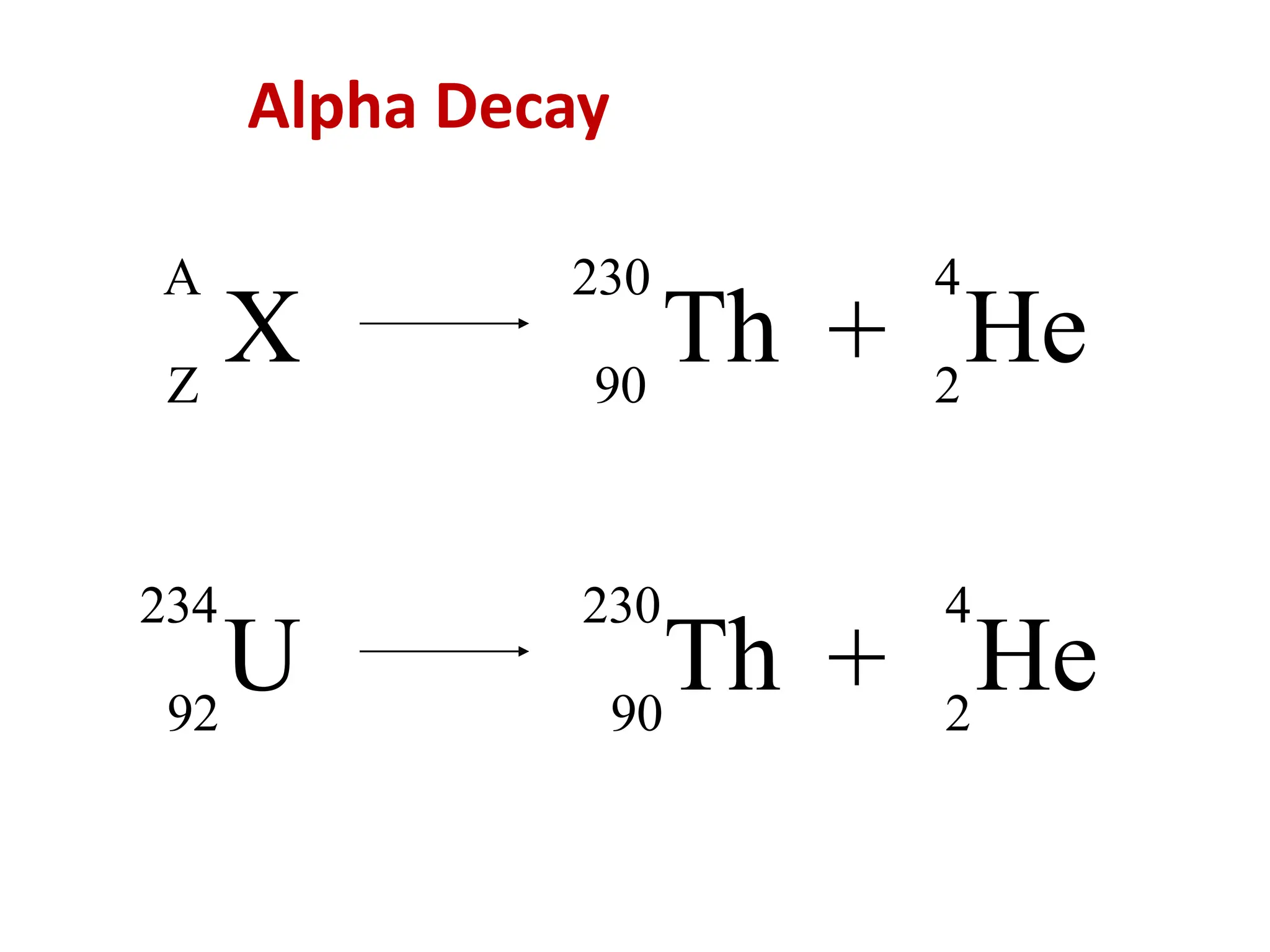
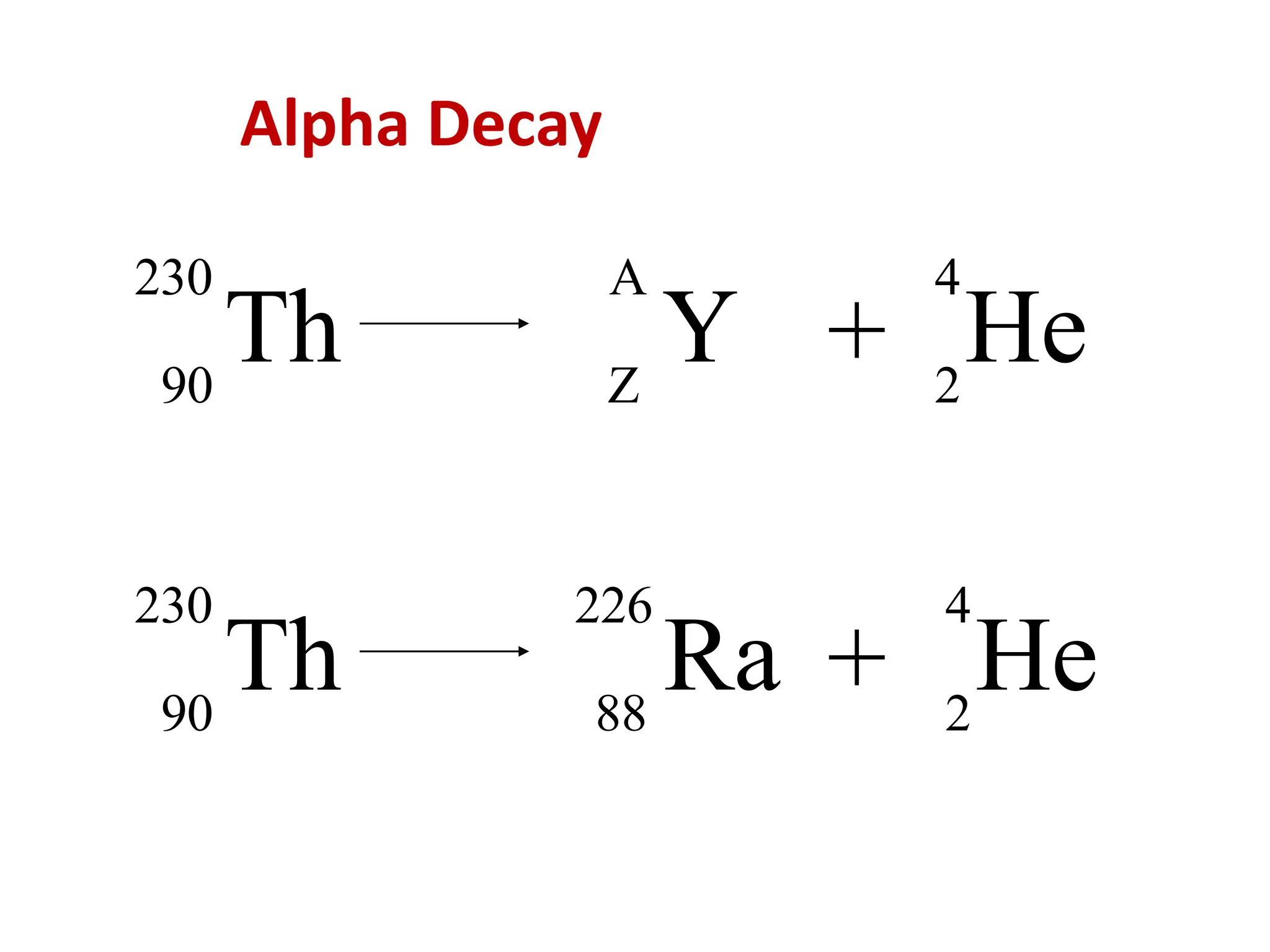
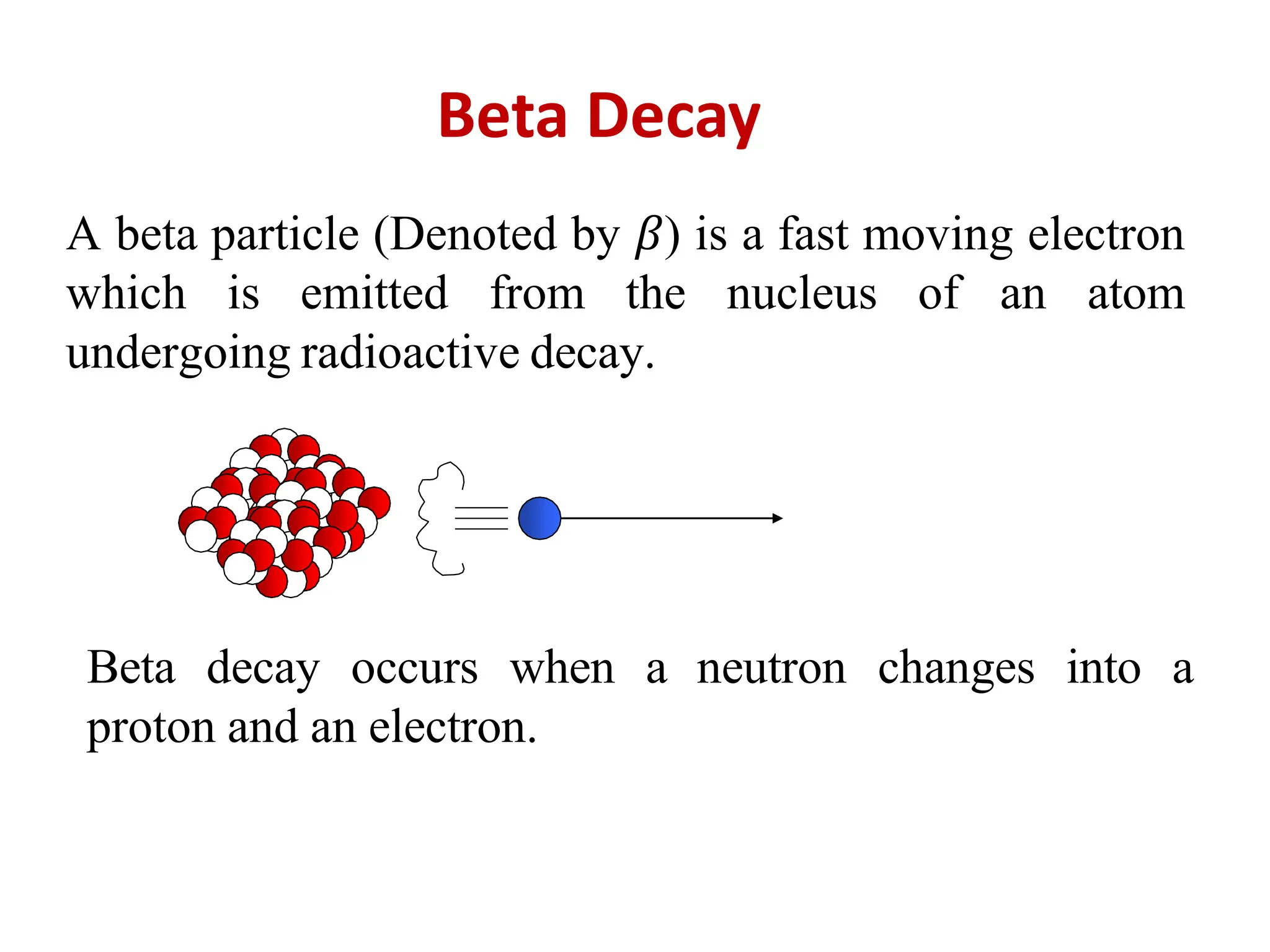
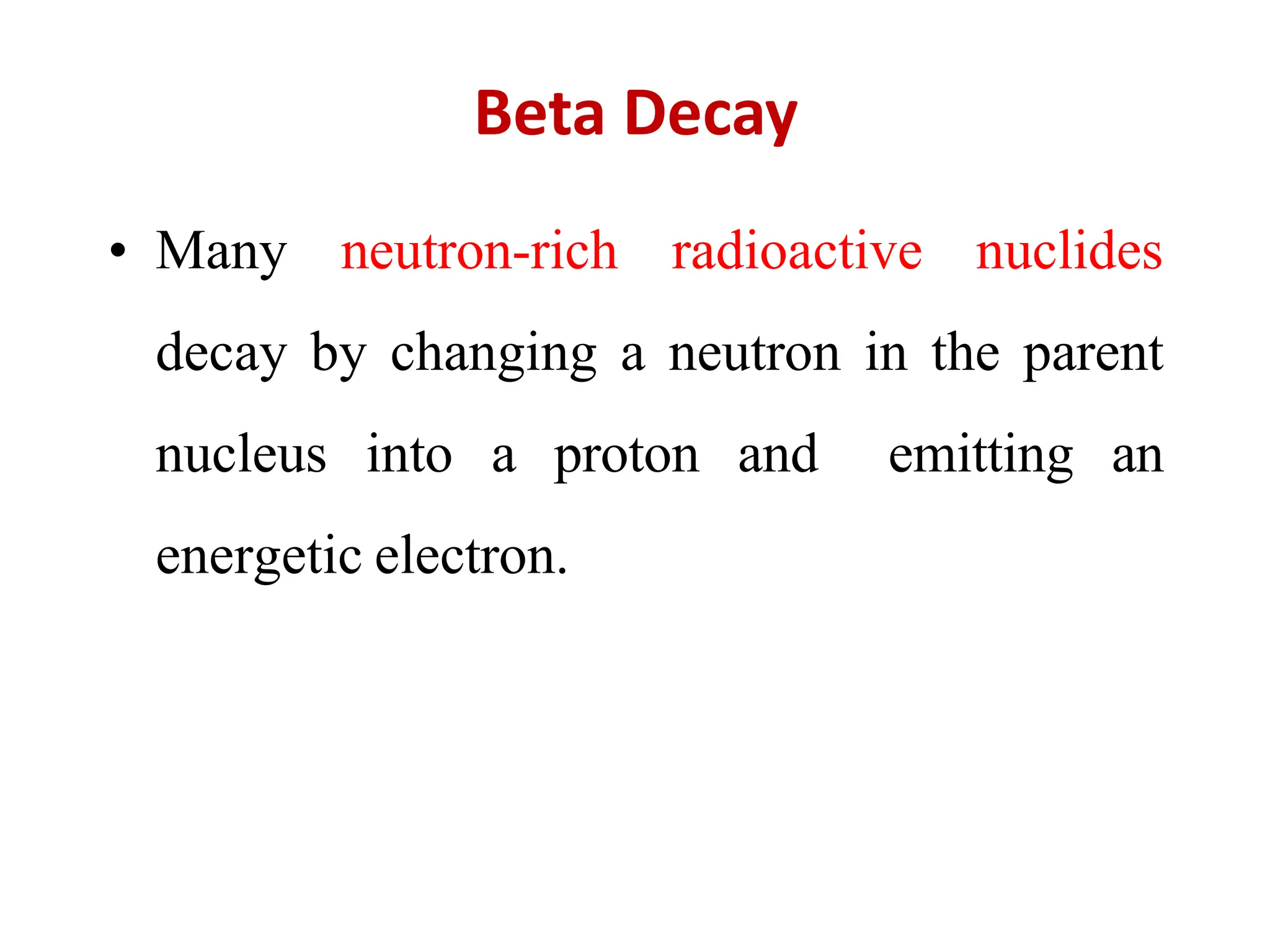
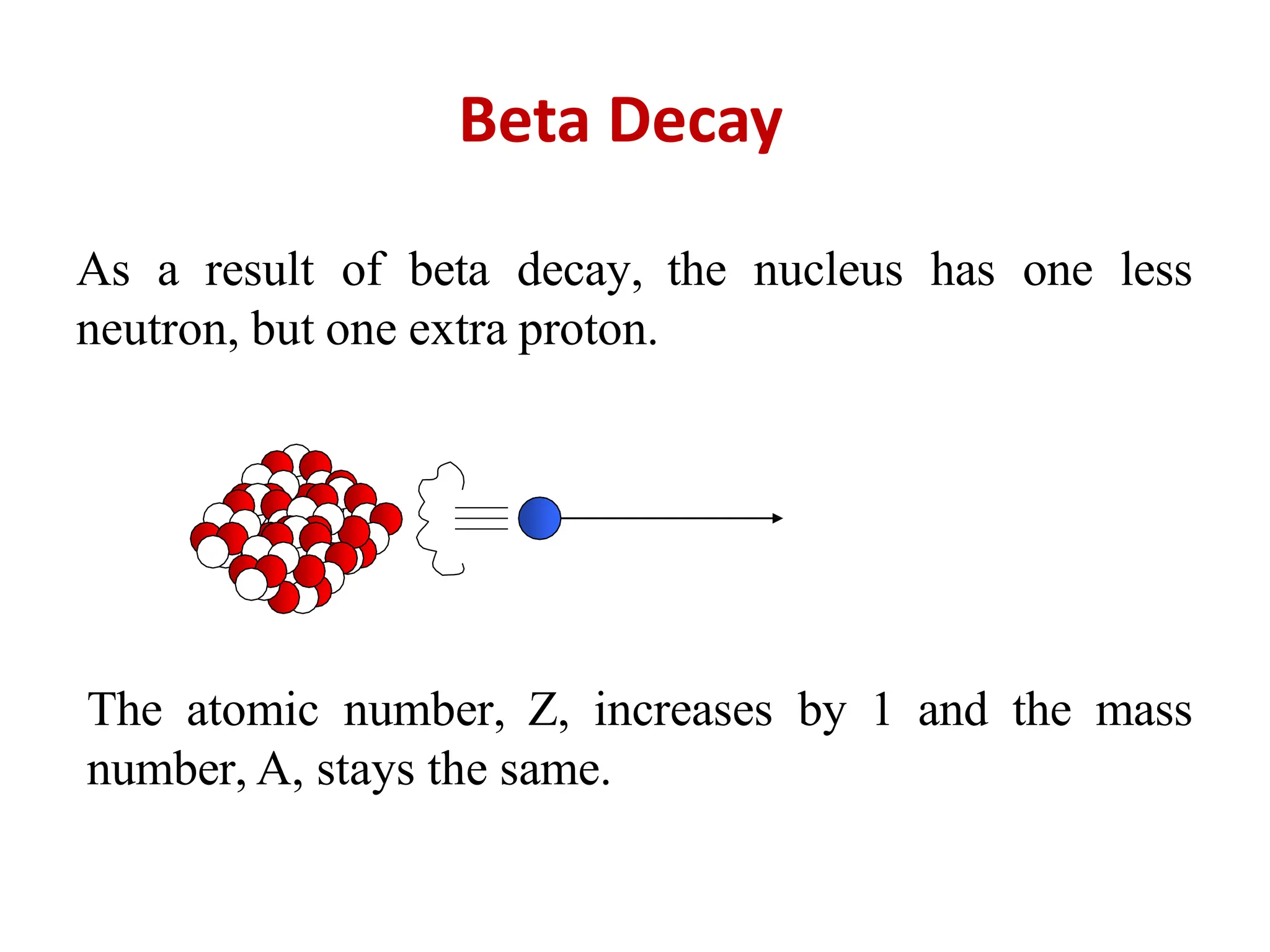
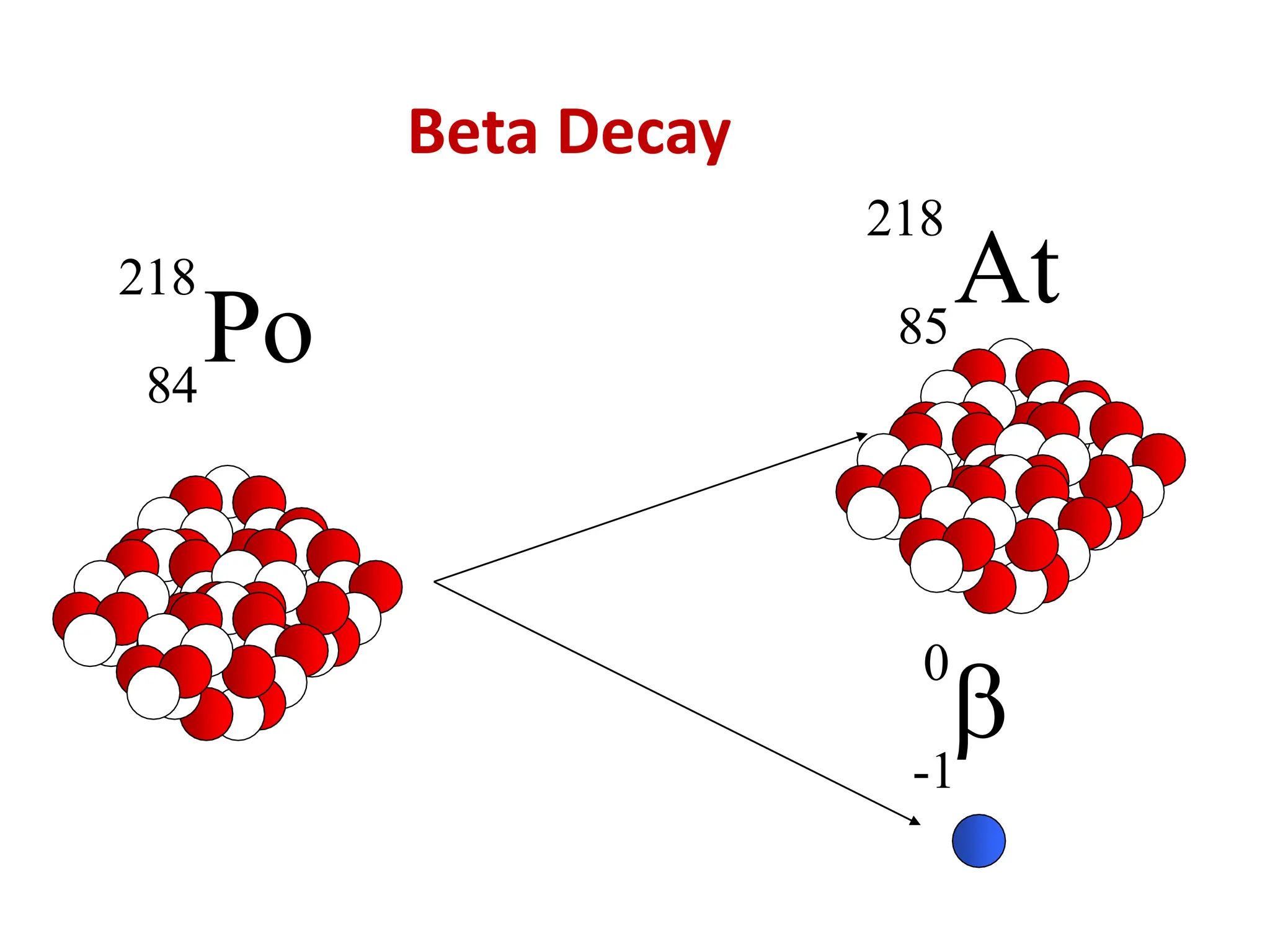
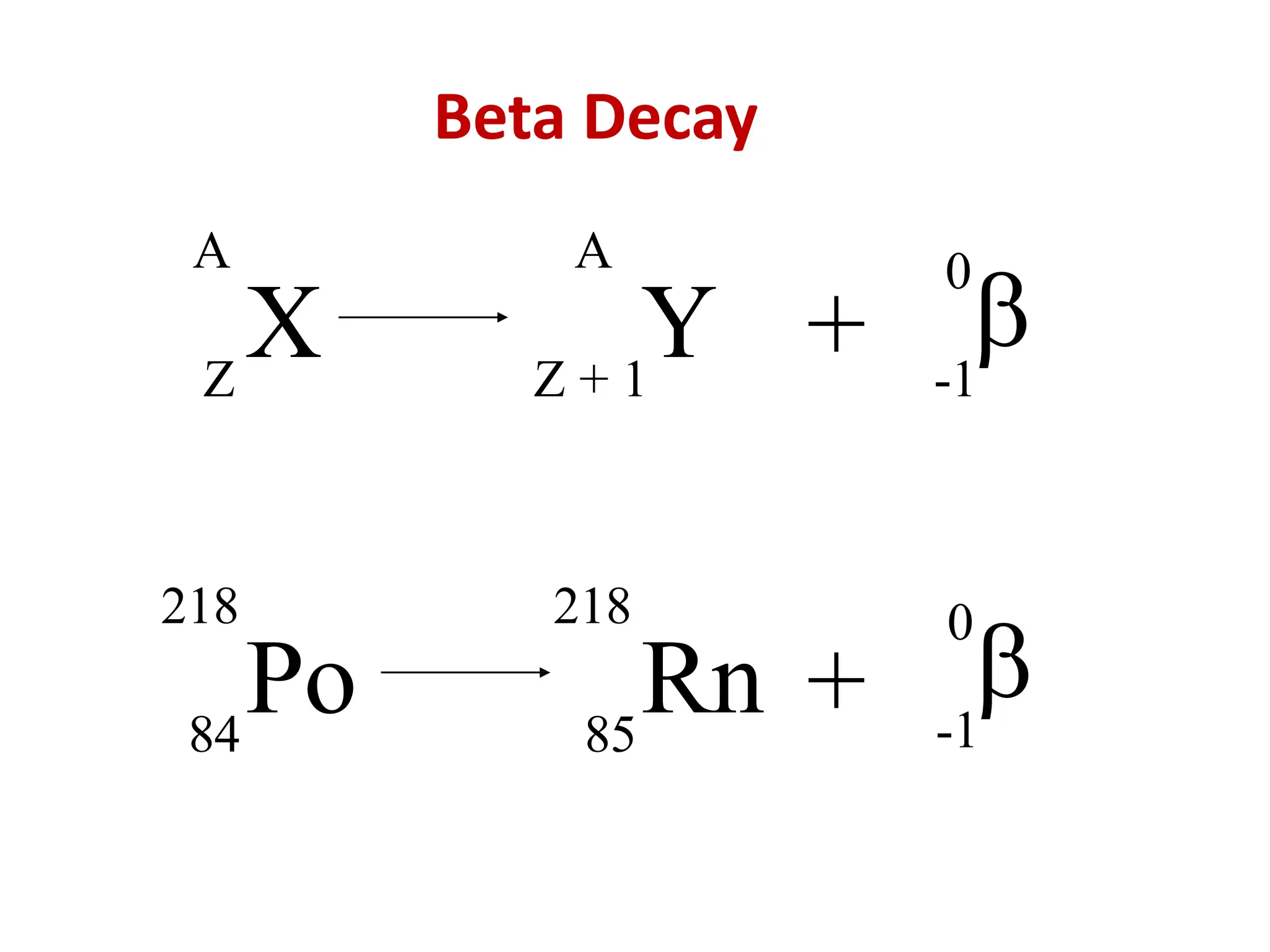
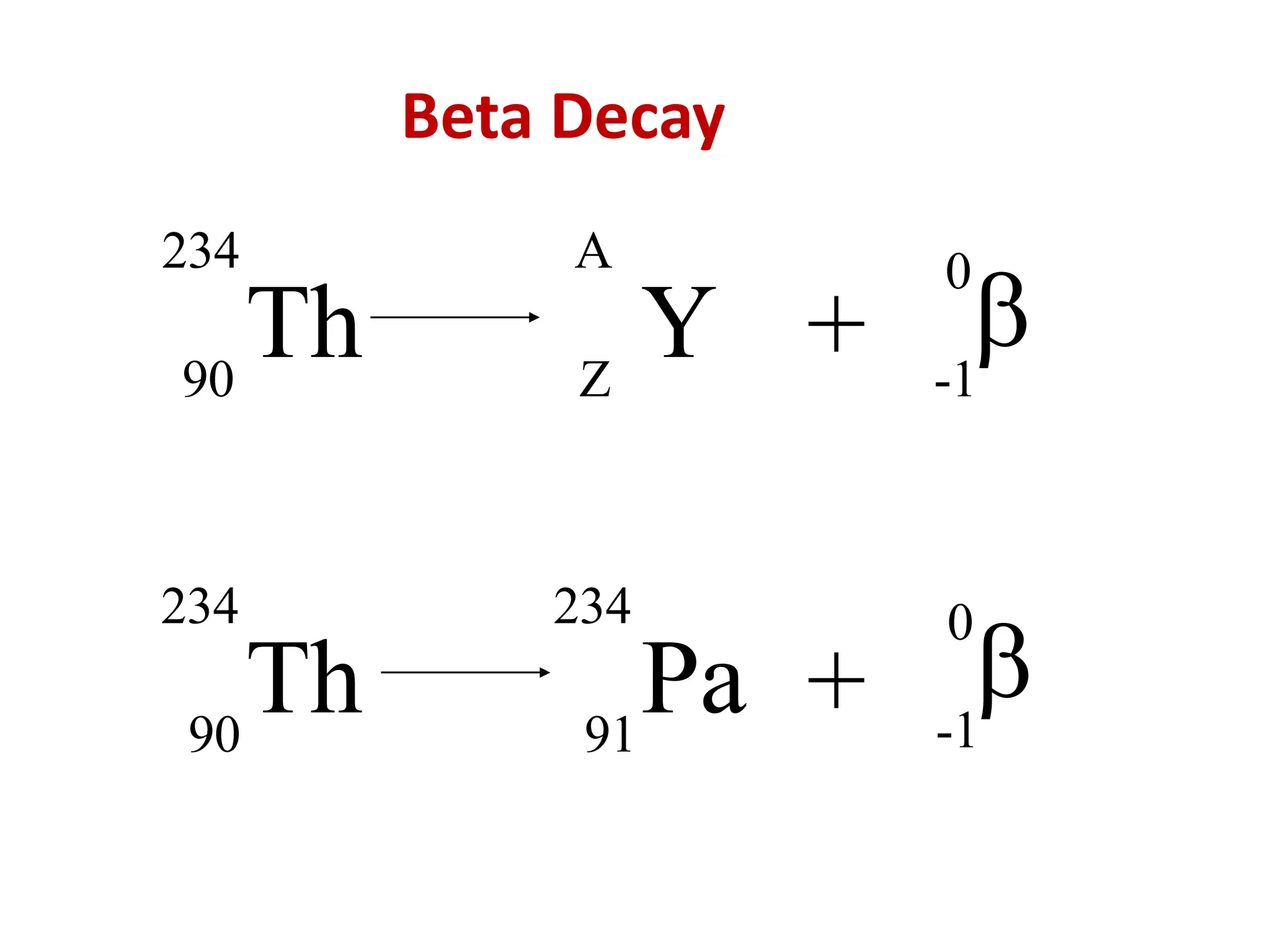
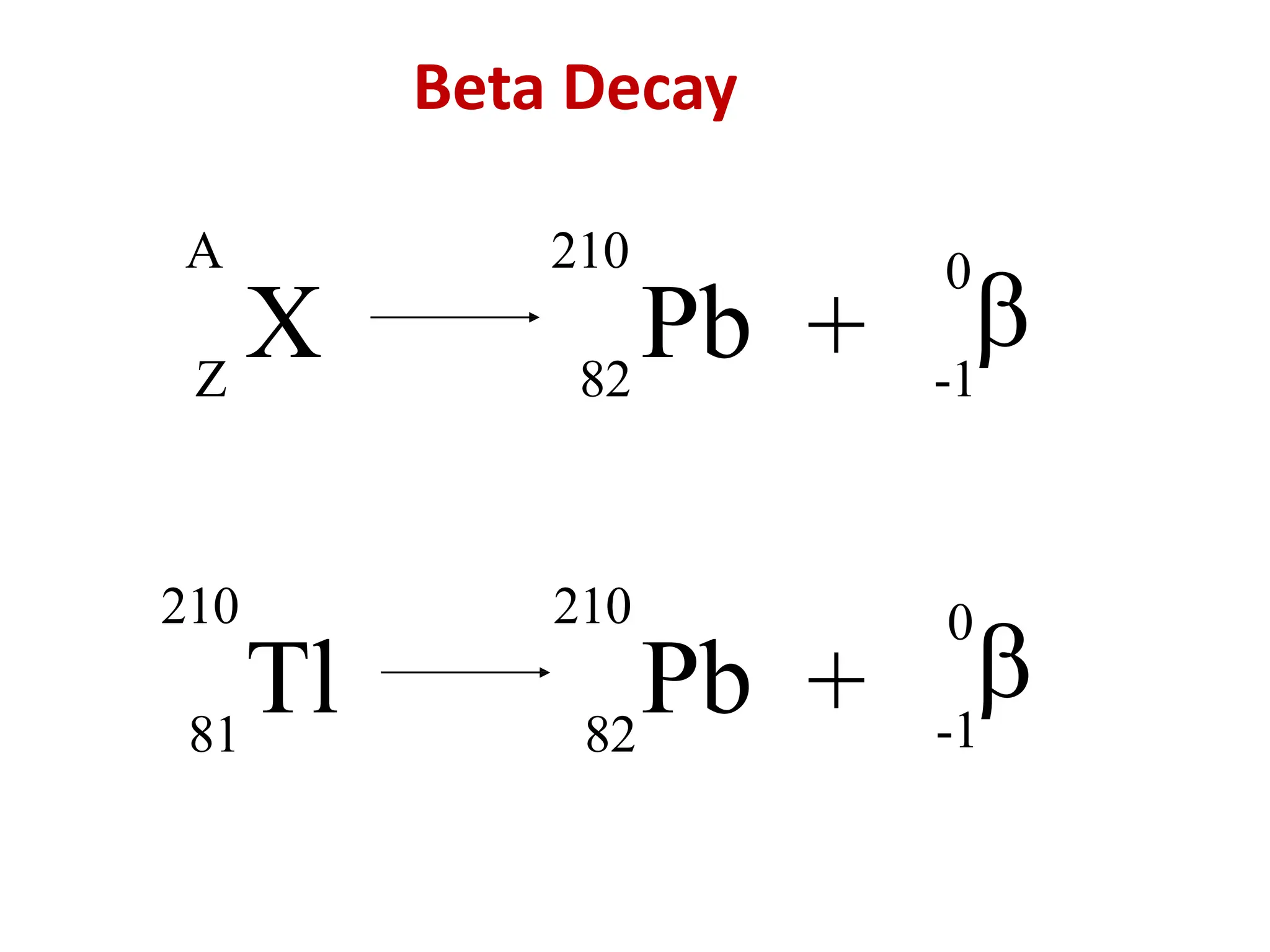
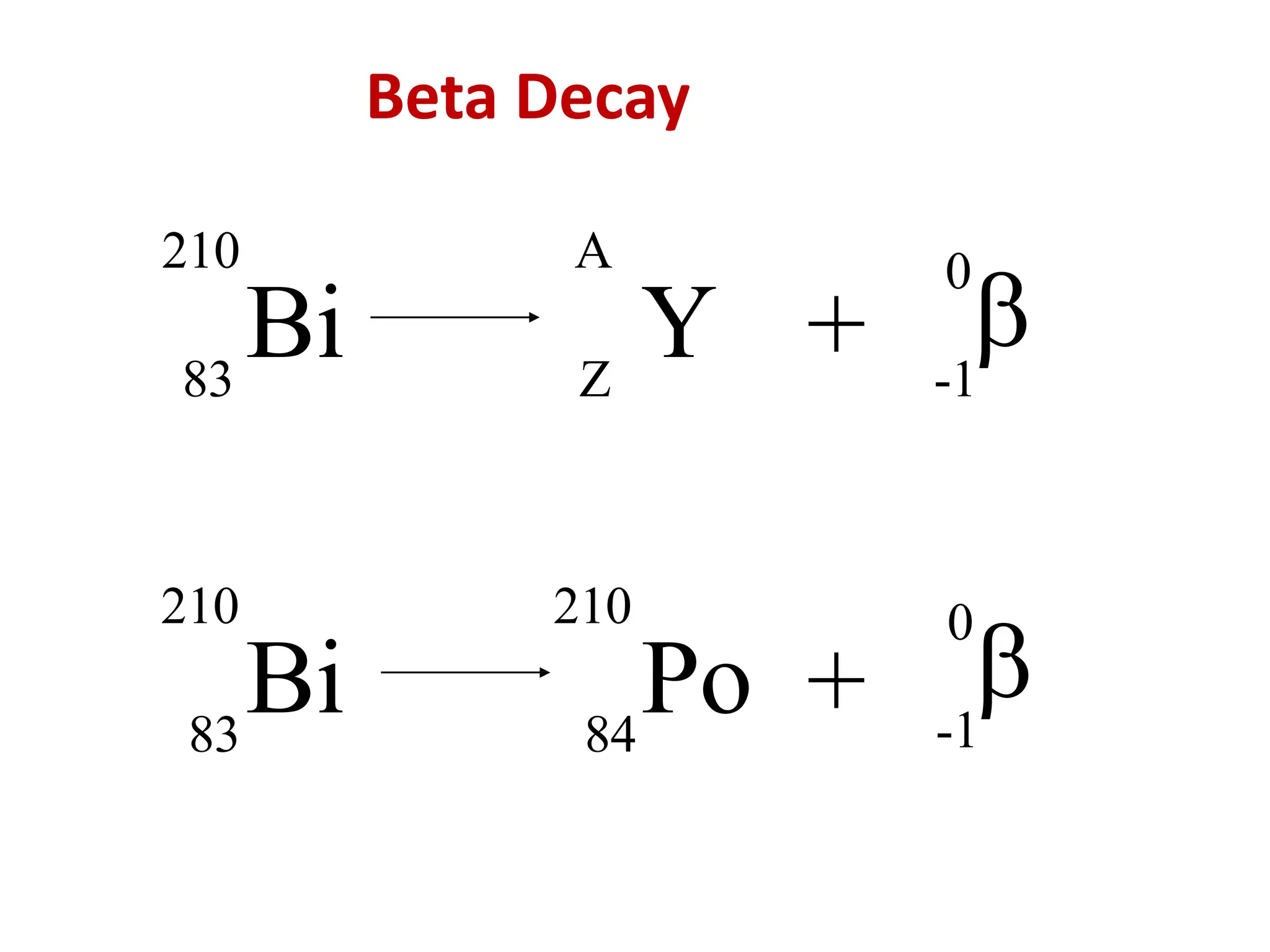
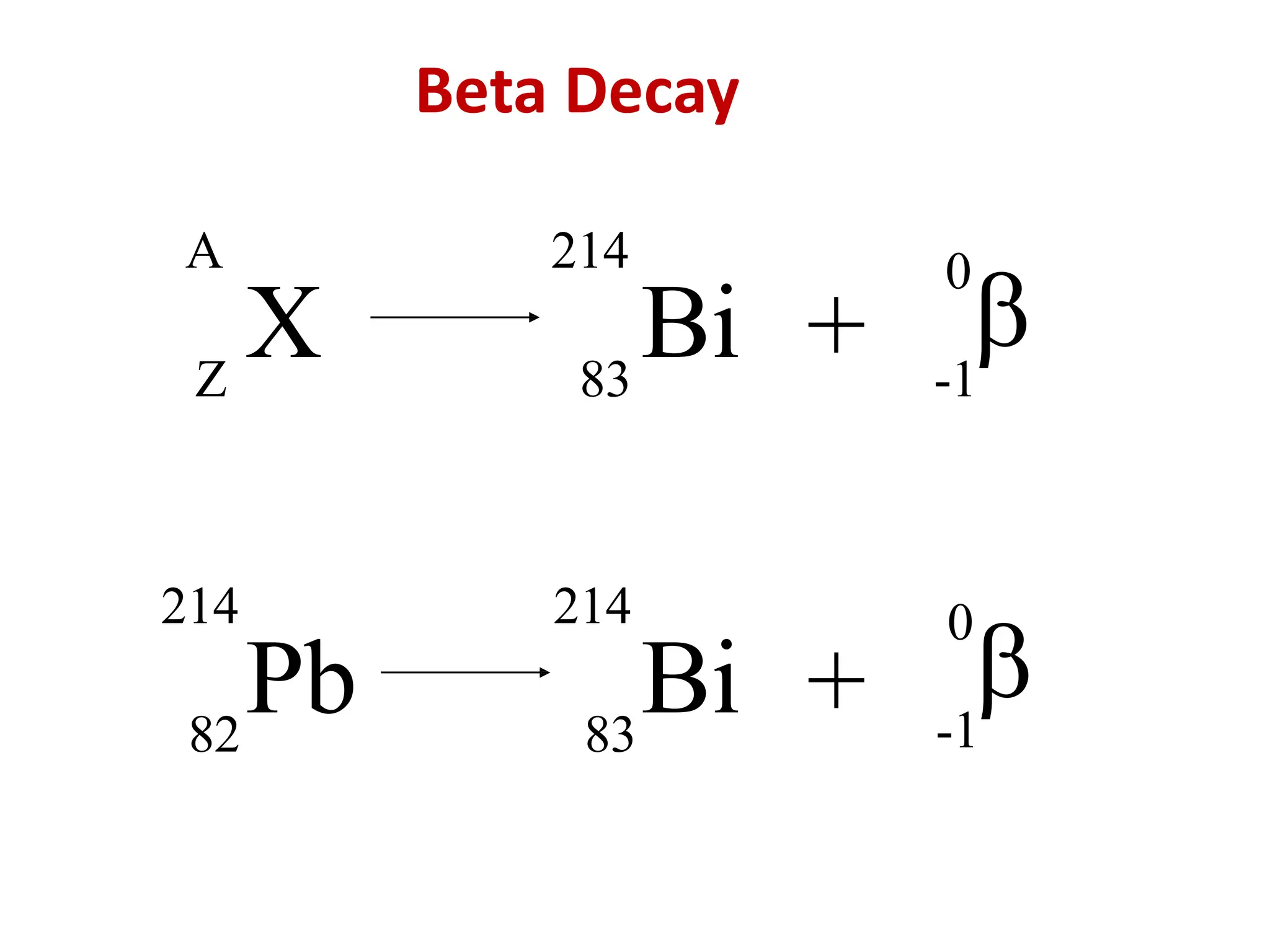
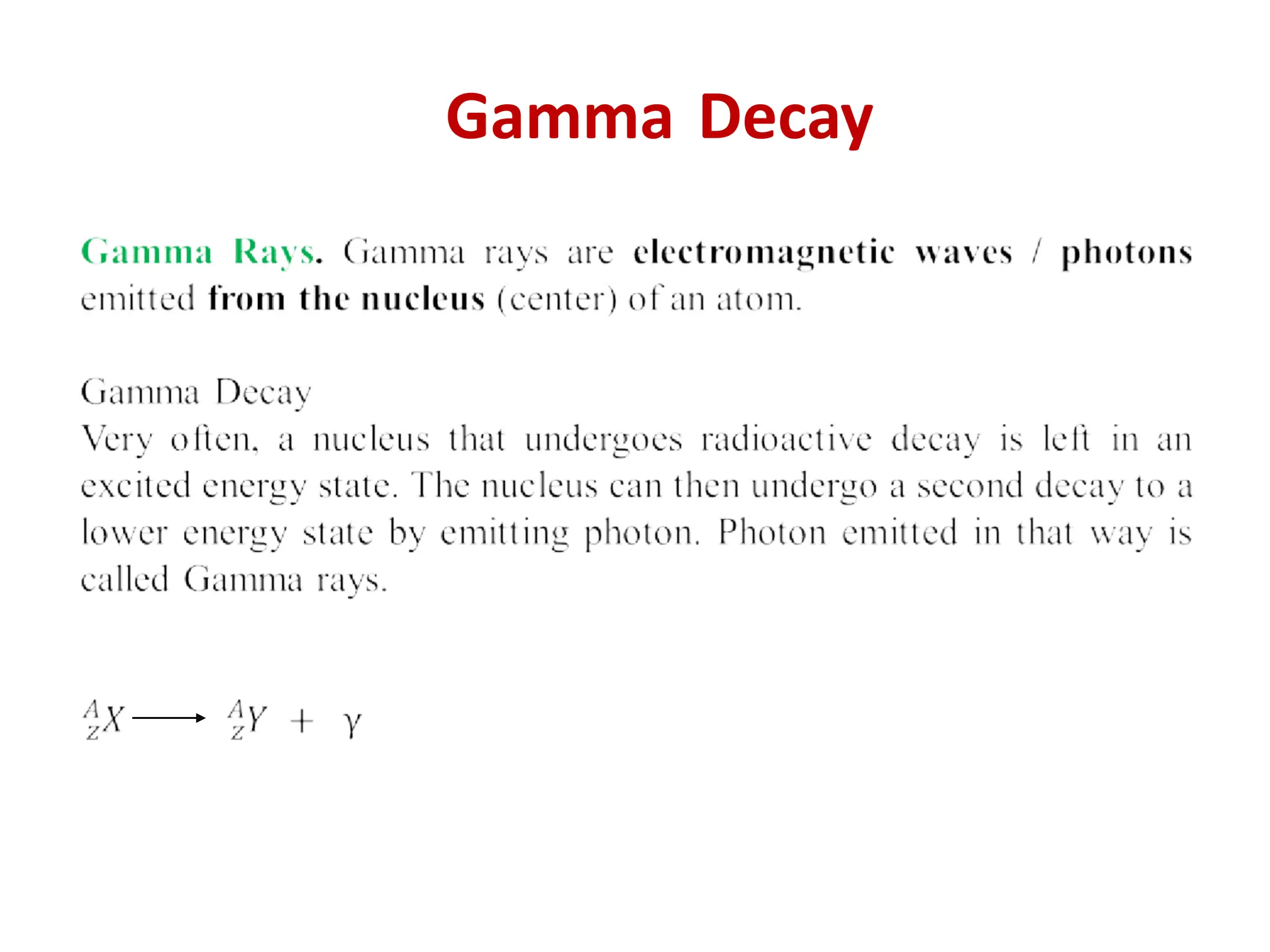
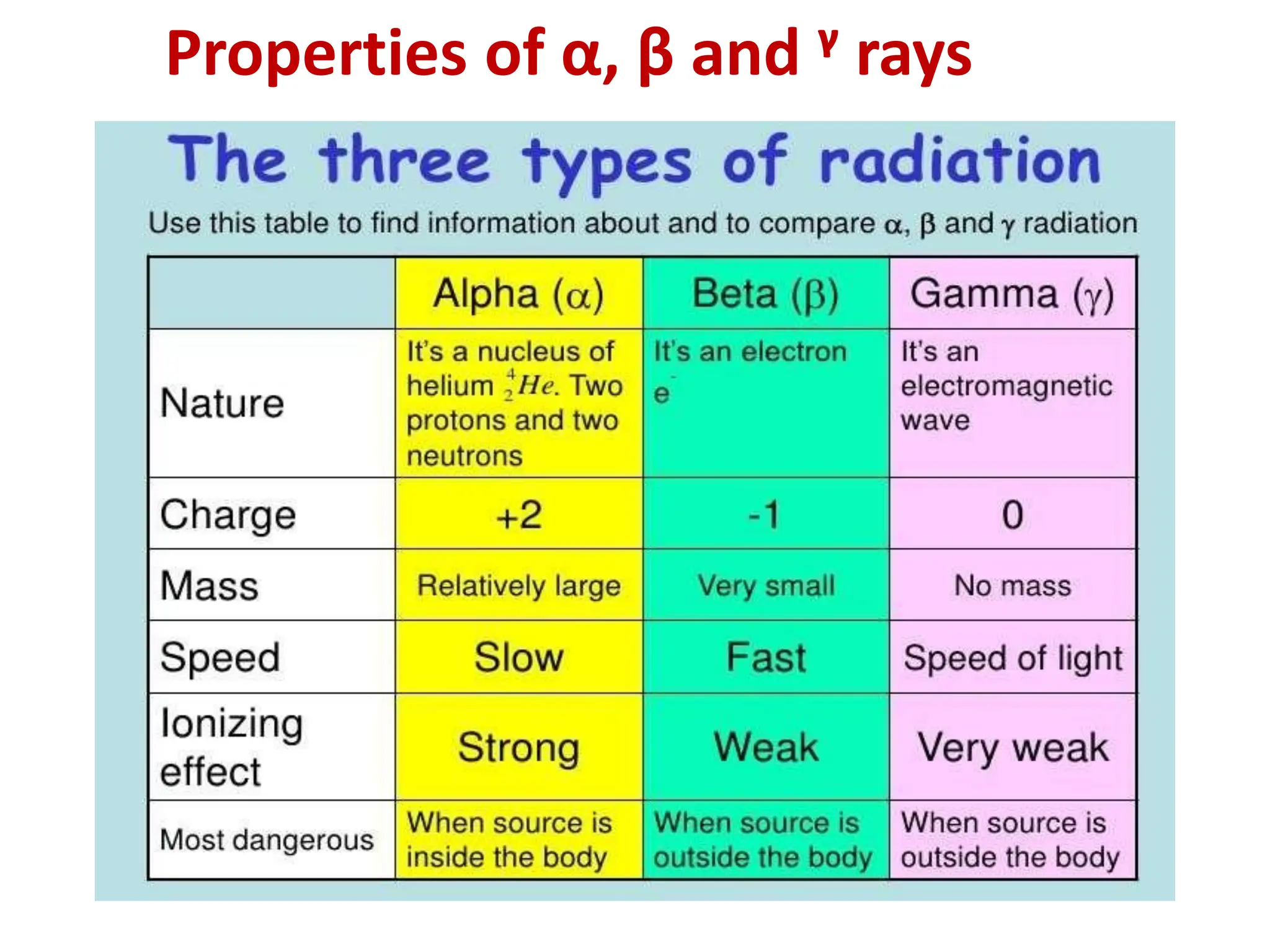
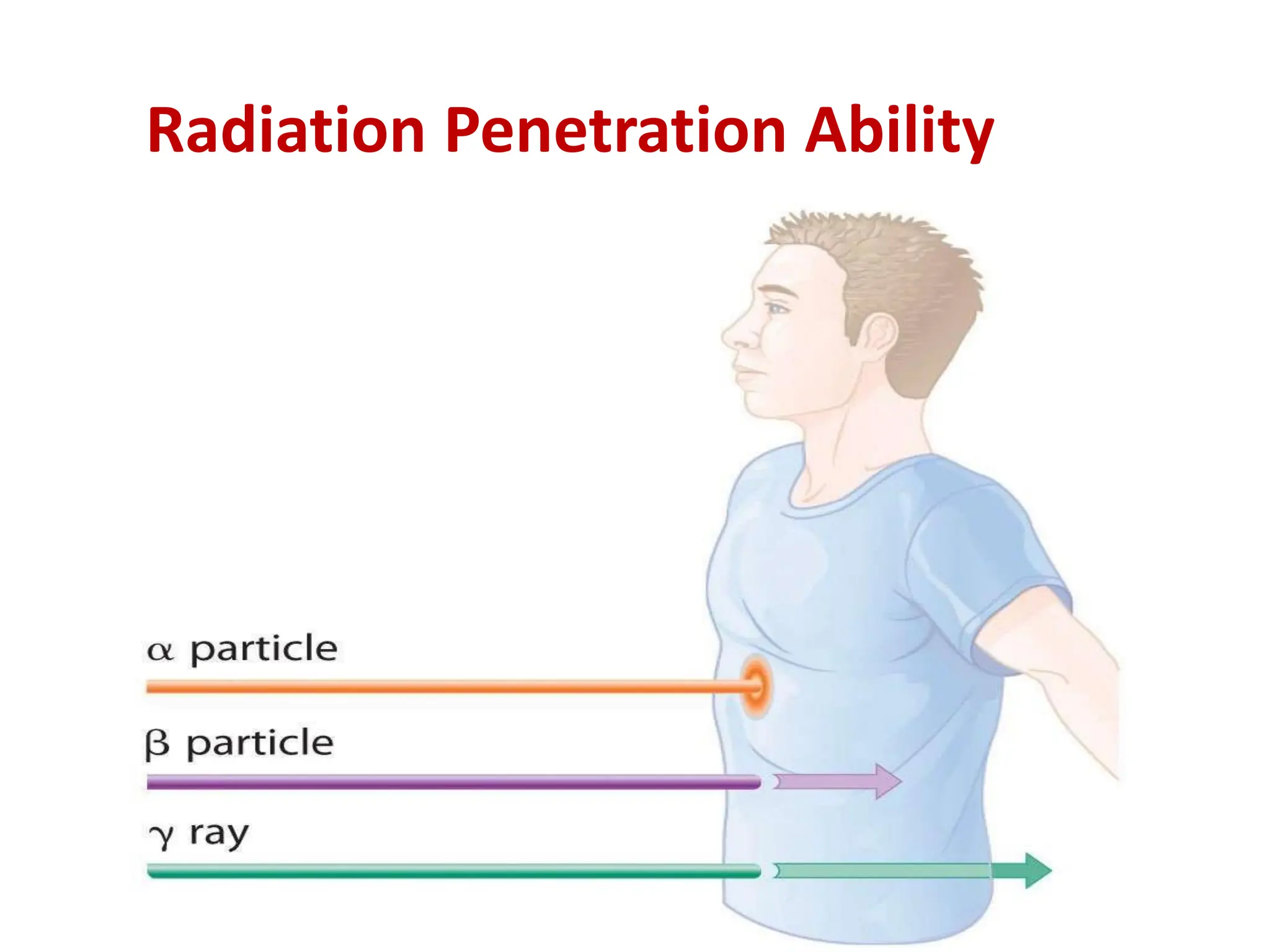
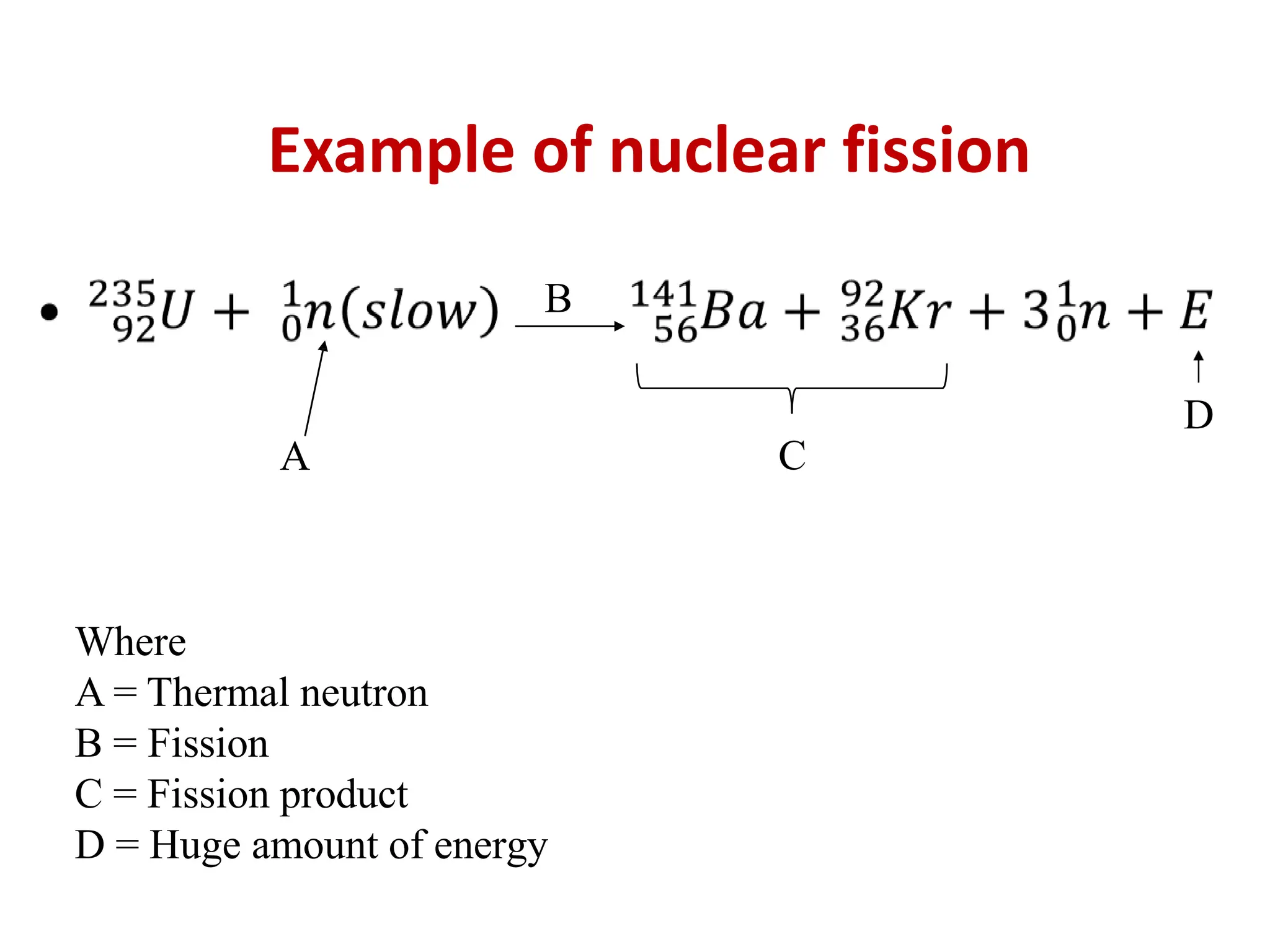
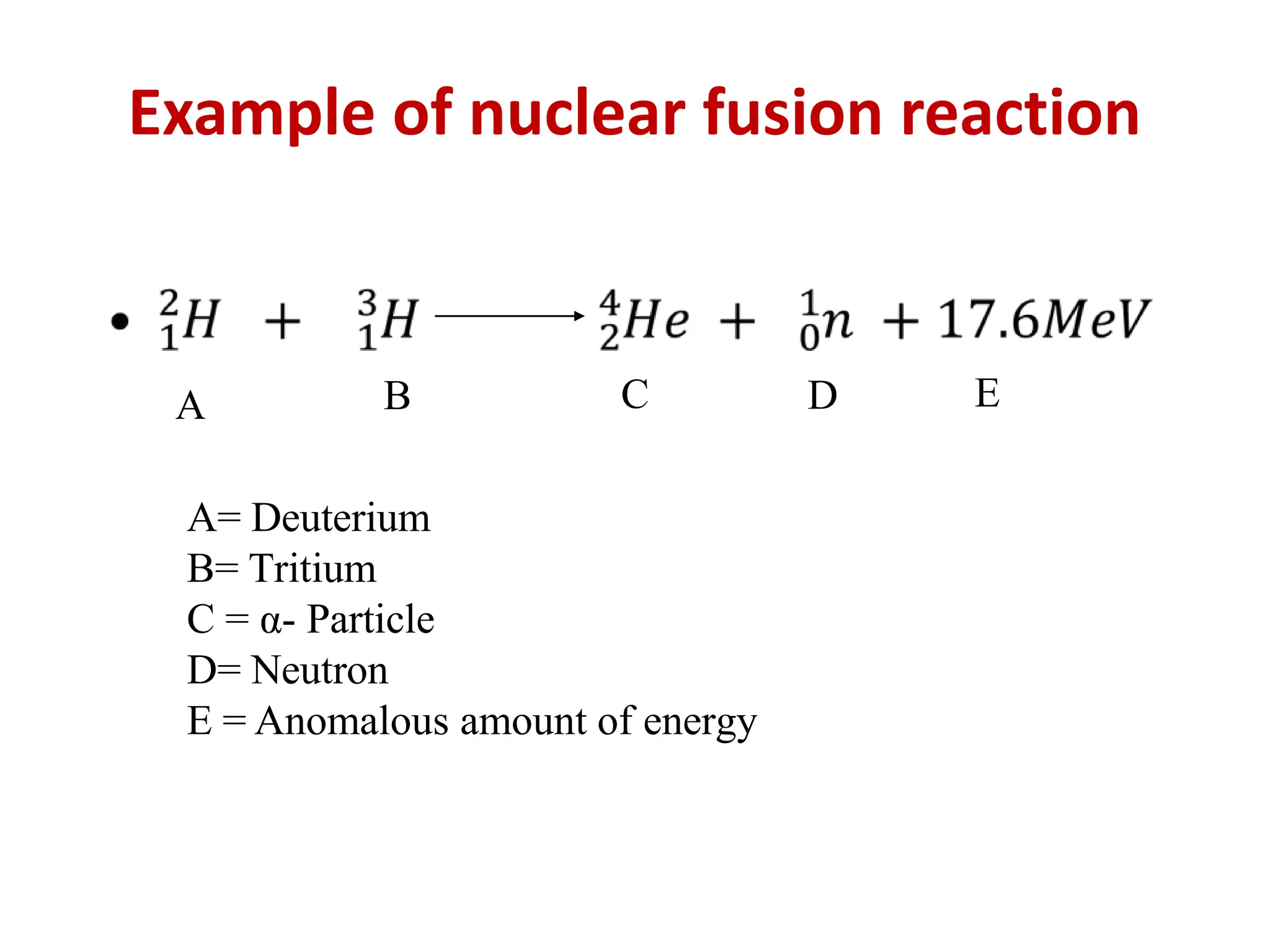
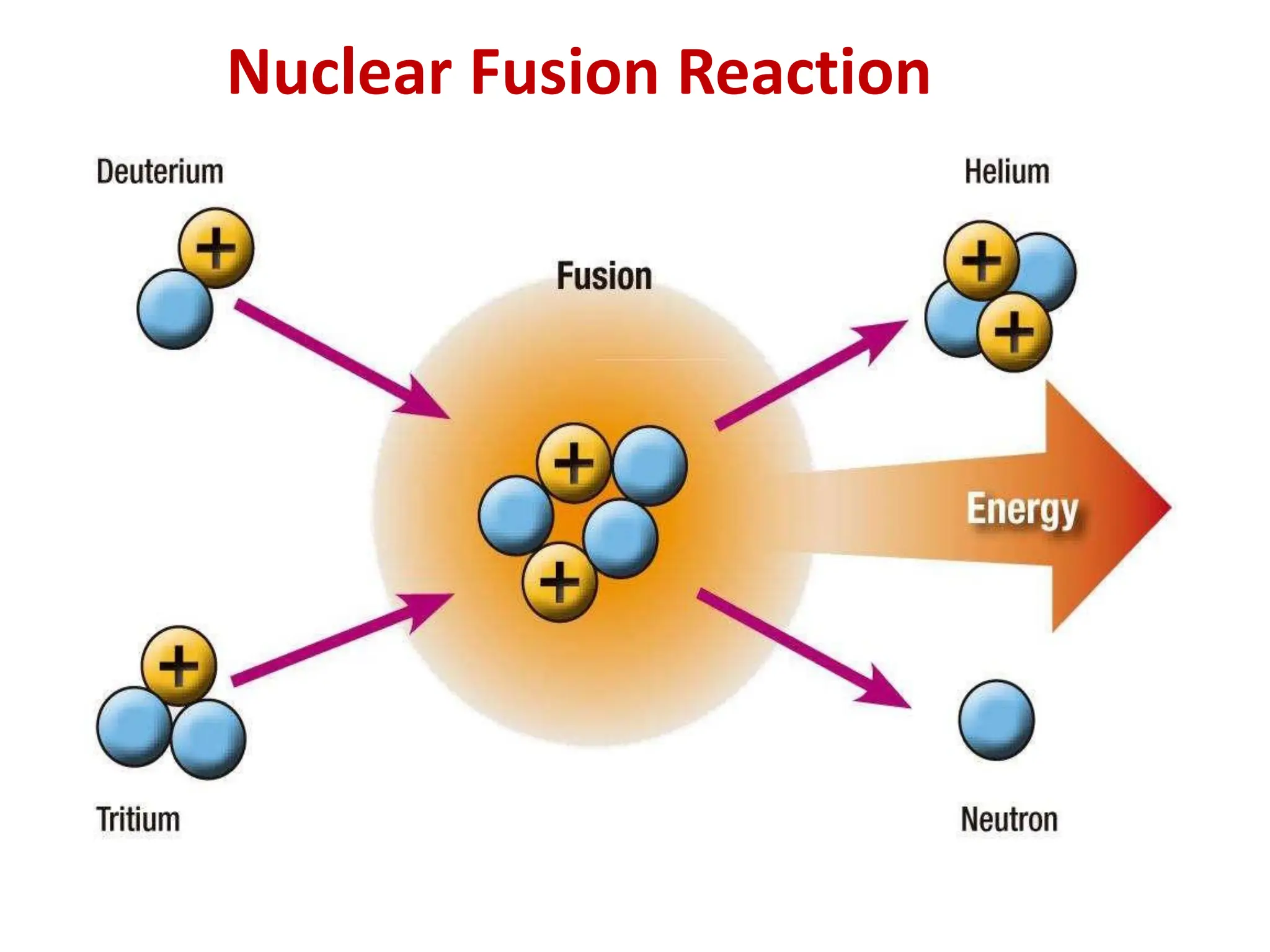
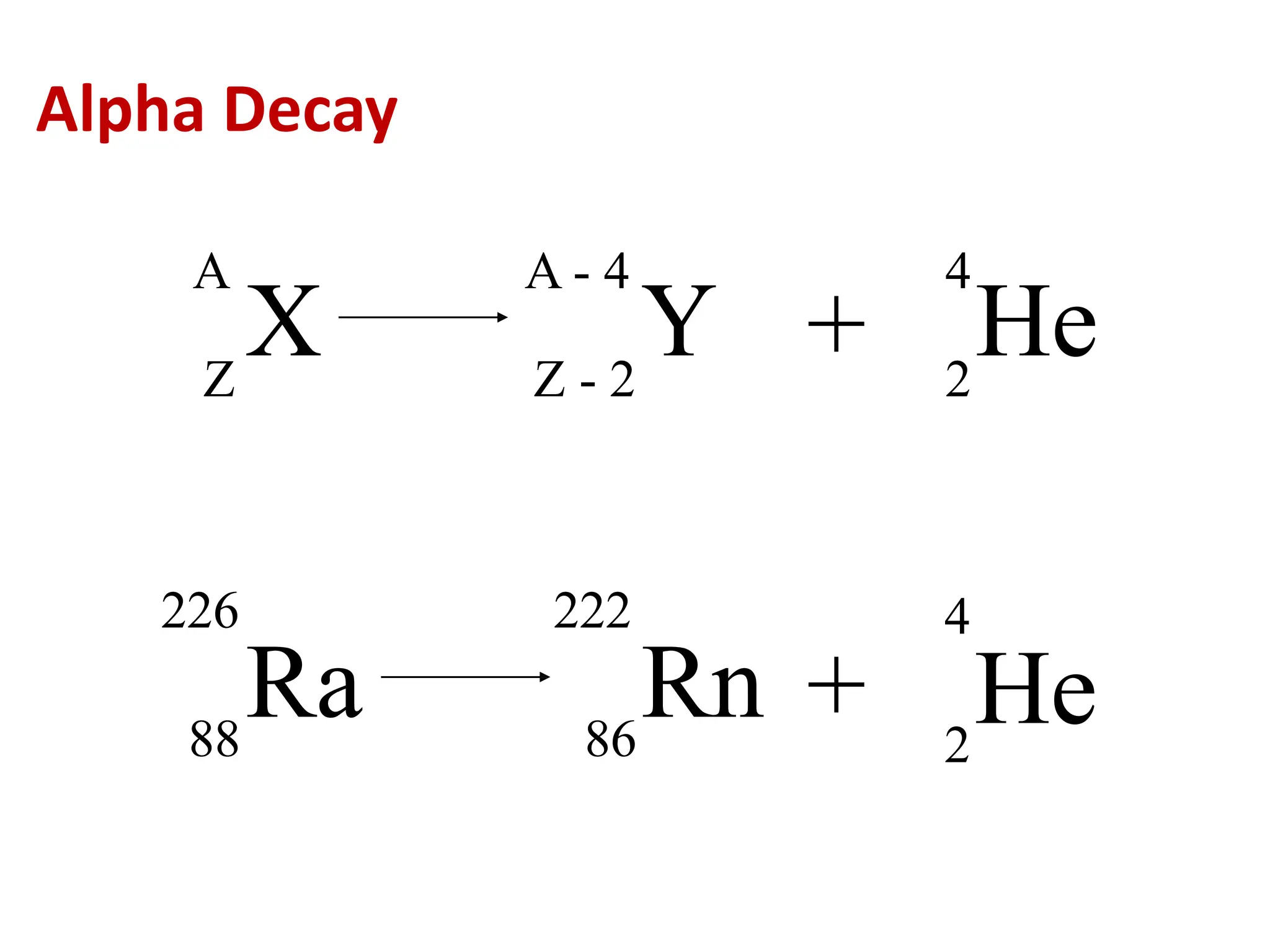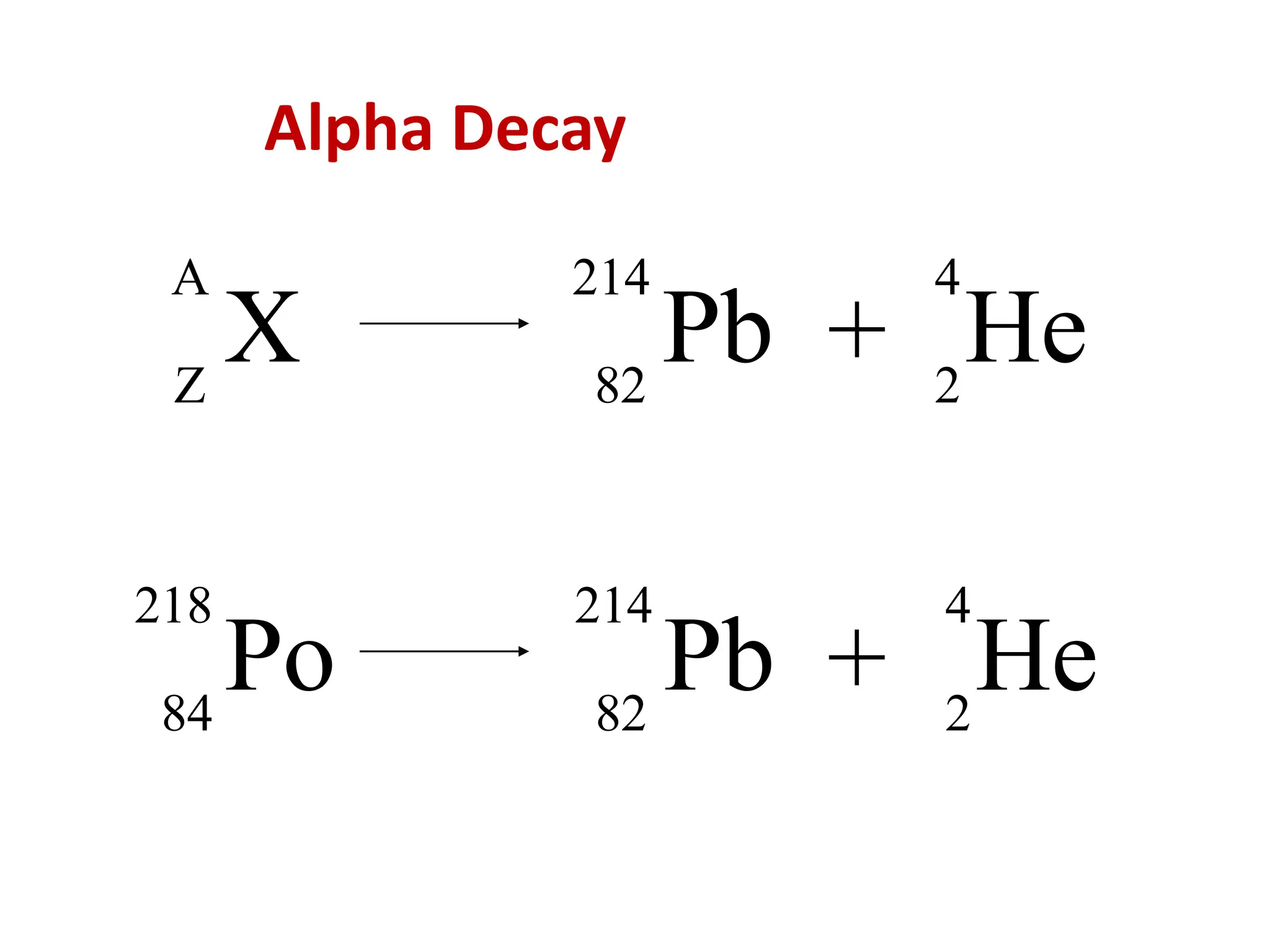The lecture discusses radioactive elements, their properties, and types of radioactive rays, including alpha (α), beta (β), and gamma (γ) rays, highlighting their mechanisms of decay and interactions with other materials. It explains radioactivity as the spontaneous disintegration of atomic nuclei leading to ionizing radiation, which poses health hazards due to its capacity to ionize atoms. Additionally, it covers nuclear reactions, detailing both nuclear fission, where heavy nuclei split, and nuclear fusion, where lighter nuclei combine to release energy.