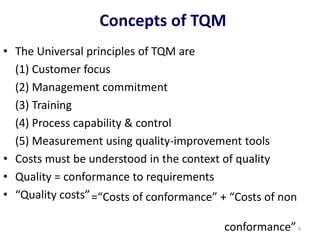
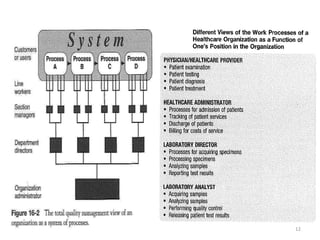
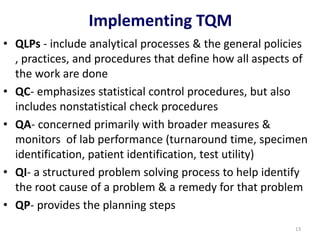
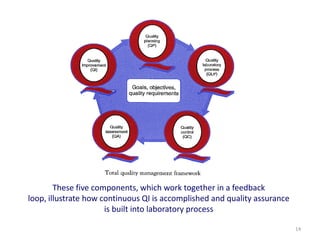
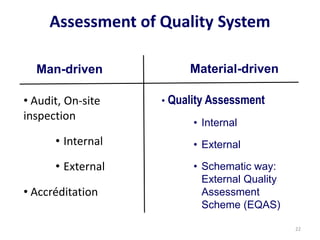
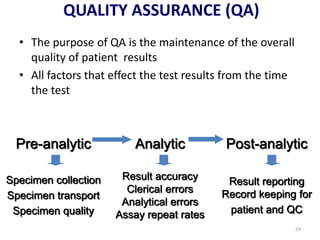
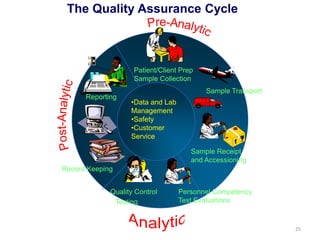
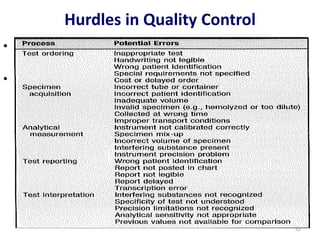
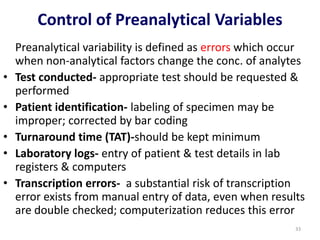
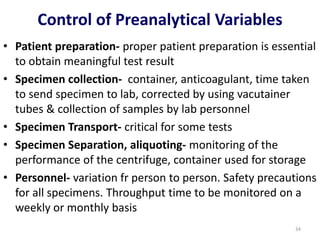
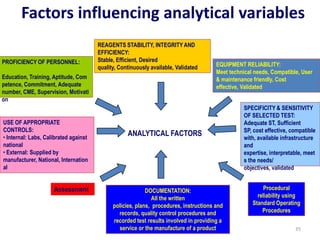
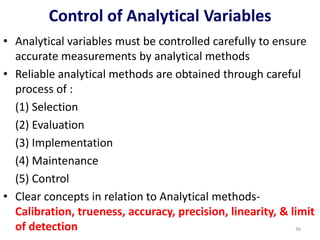
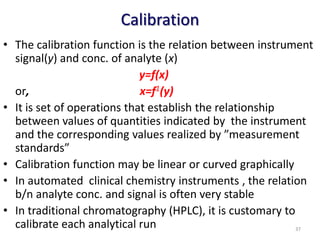
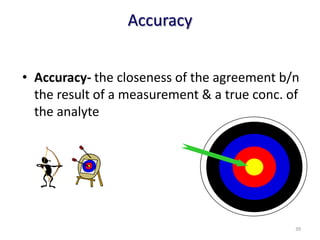
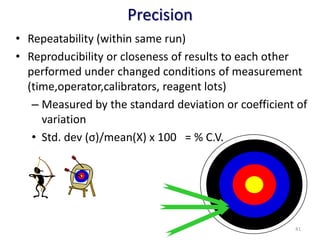
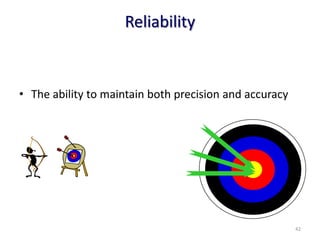
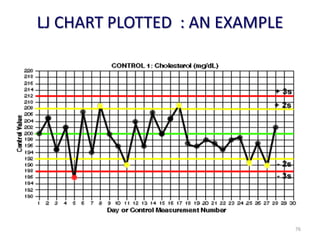
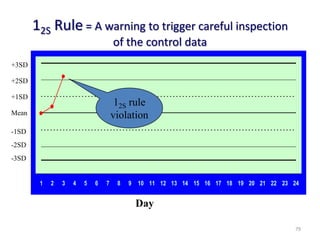
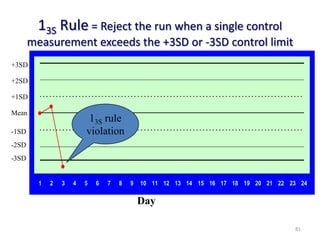
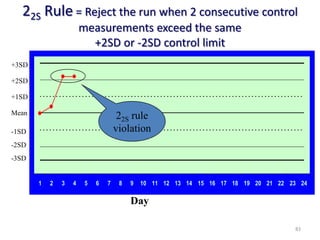
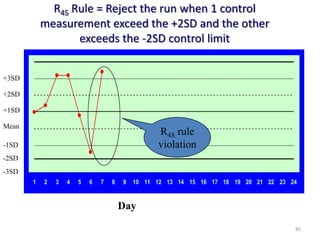
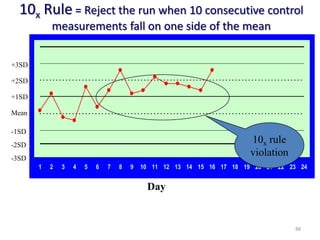
The document outlines the importance of quality control in clinical laboratories, detailing concepts such as total quality management (TQM), quality assurance (QA), and the necessity for continuous quality improvement (CQI). It emphasizes the need for robust testing systems, the role of laboratory staff in quality assurance, and the significance of proper documentation and standard operating procedures (SOPs). Overall, the document stresses that quality in labs is a collective responsibility crucial for providing reliable and accurate test results, which ultimately impacts patient care.