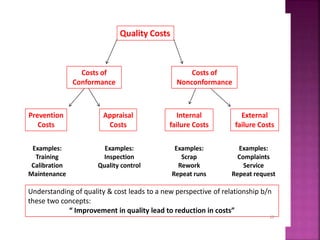
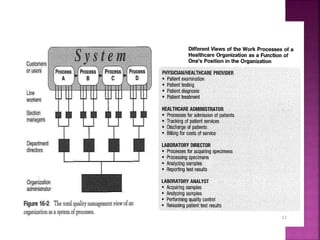
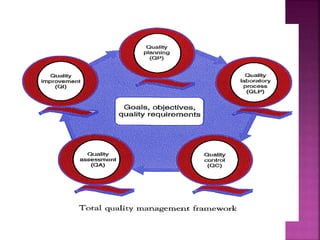
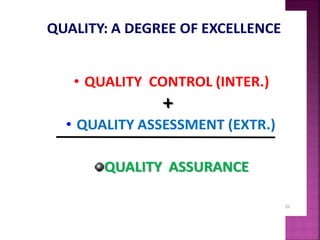
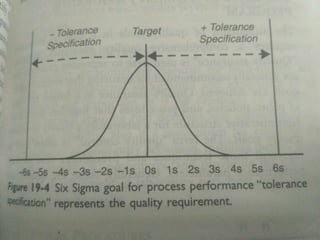
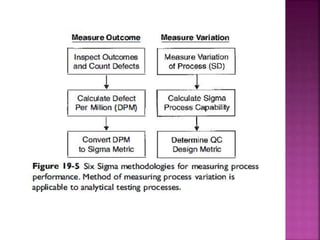
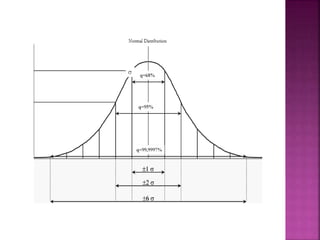
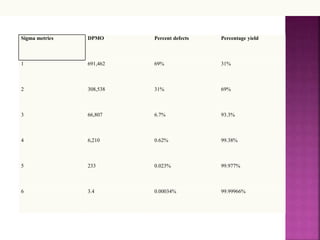
This document discusses quality management concepts in healthcare laboratories. It defines key terms like quality, quality assurance, quality control, total quality management. It explains approaches like continuous quality improvement, quality assessment and sigma metrics that are used to monitor performance and ensure reliable test results. The goal of quality management is to deliver accurate and timely reports to healthcare providers and continuously improve laboratory processes.