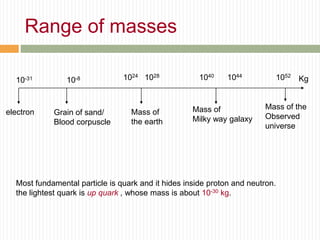
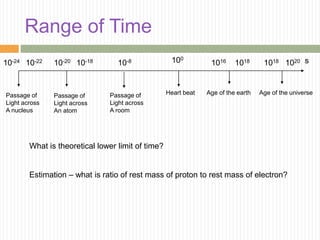
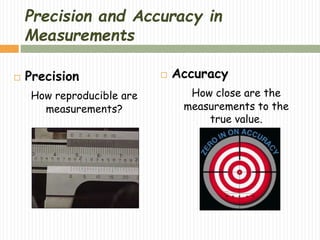
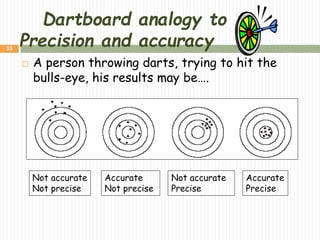
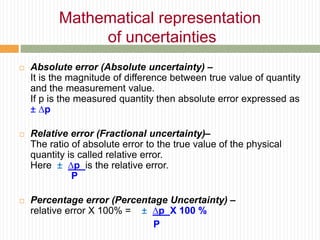
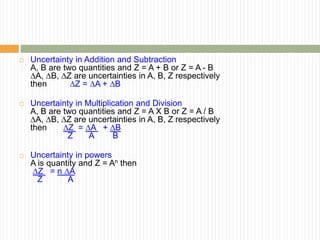
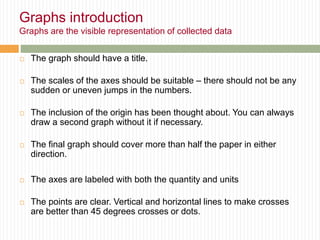
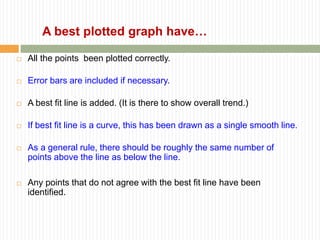
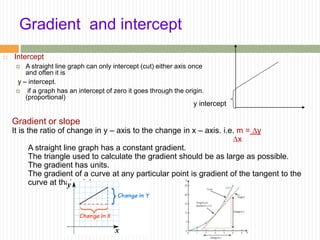
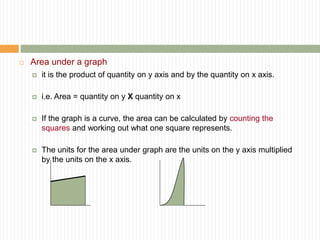
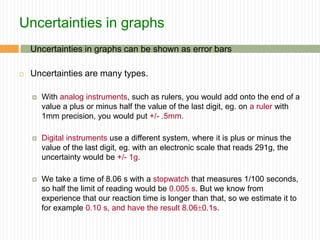
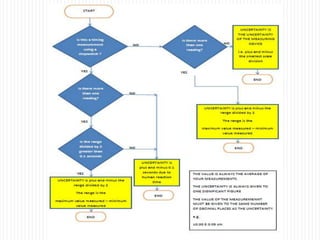
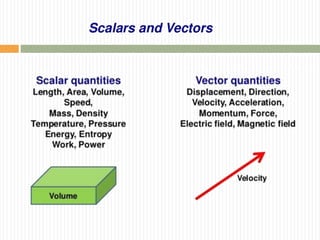
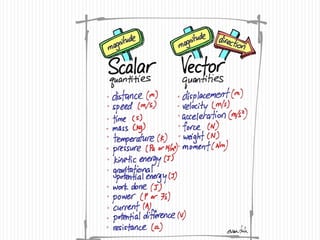
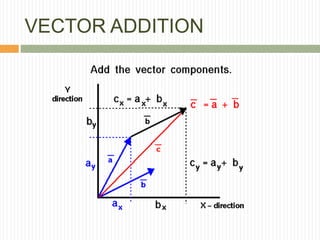
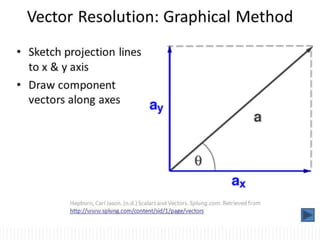
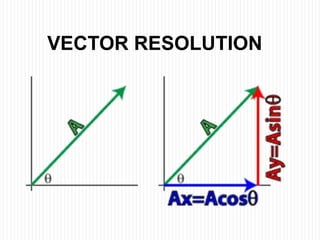
This document provides information about measurement and uncertainty in physics. It defines key terms like physical quantities, units, and order of magnitude. It discusses the International System of Units and its seven base units. The document also covers topics like derived units, significant figures, types of errors, calculating uncertainty, and basics of graphing collected data.