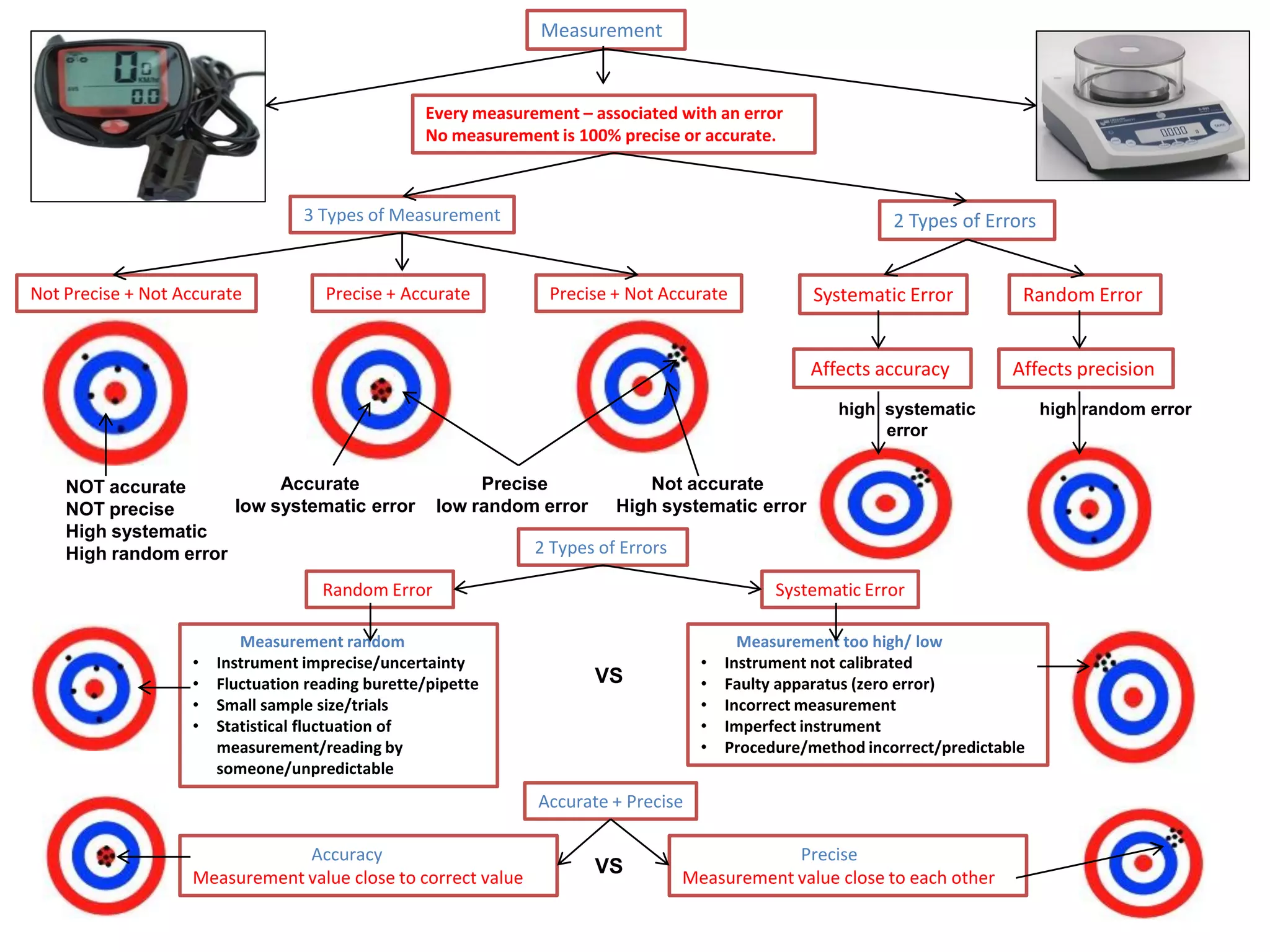
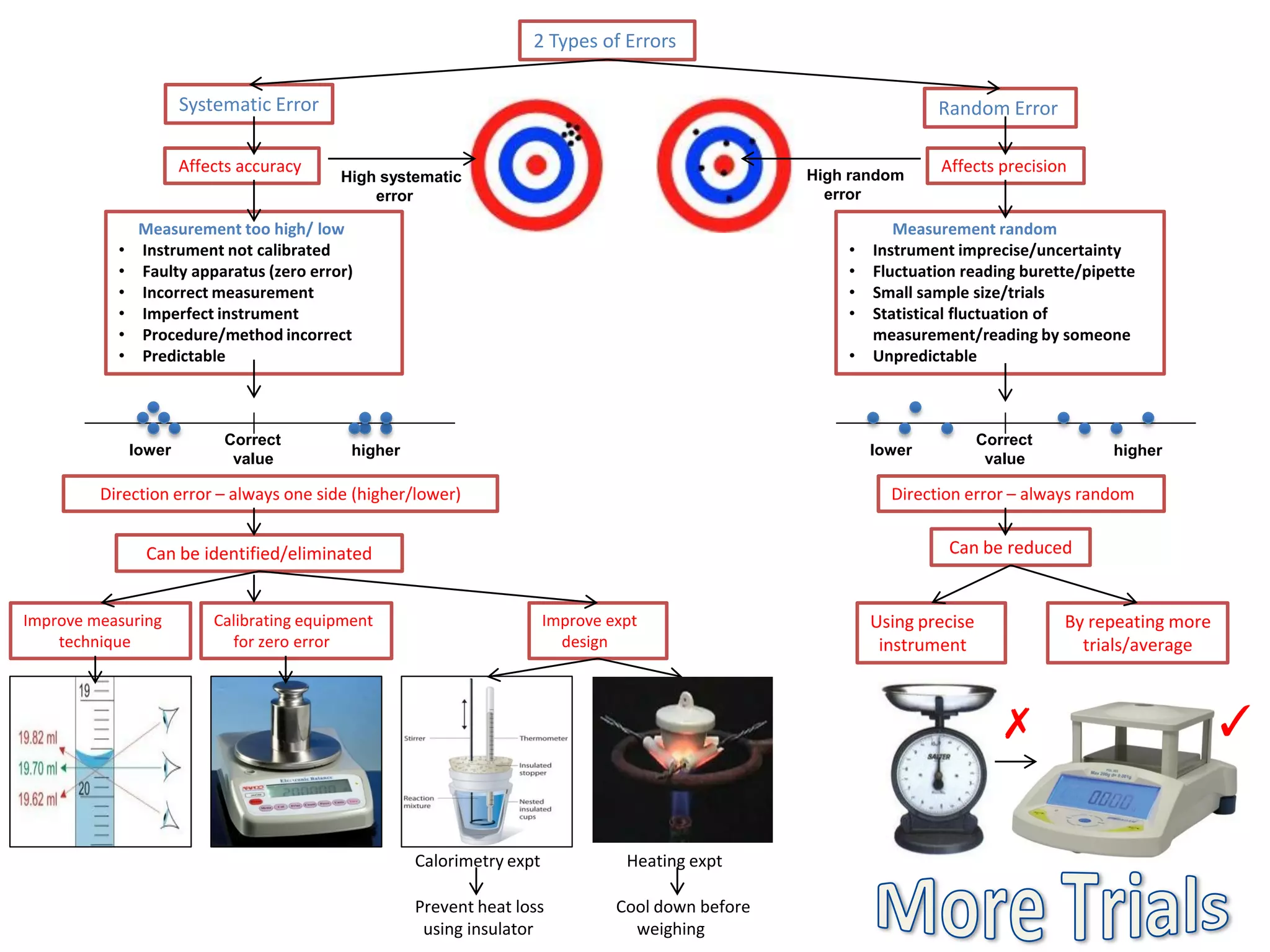
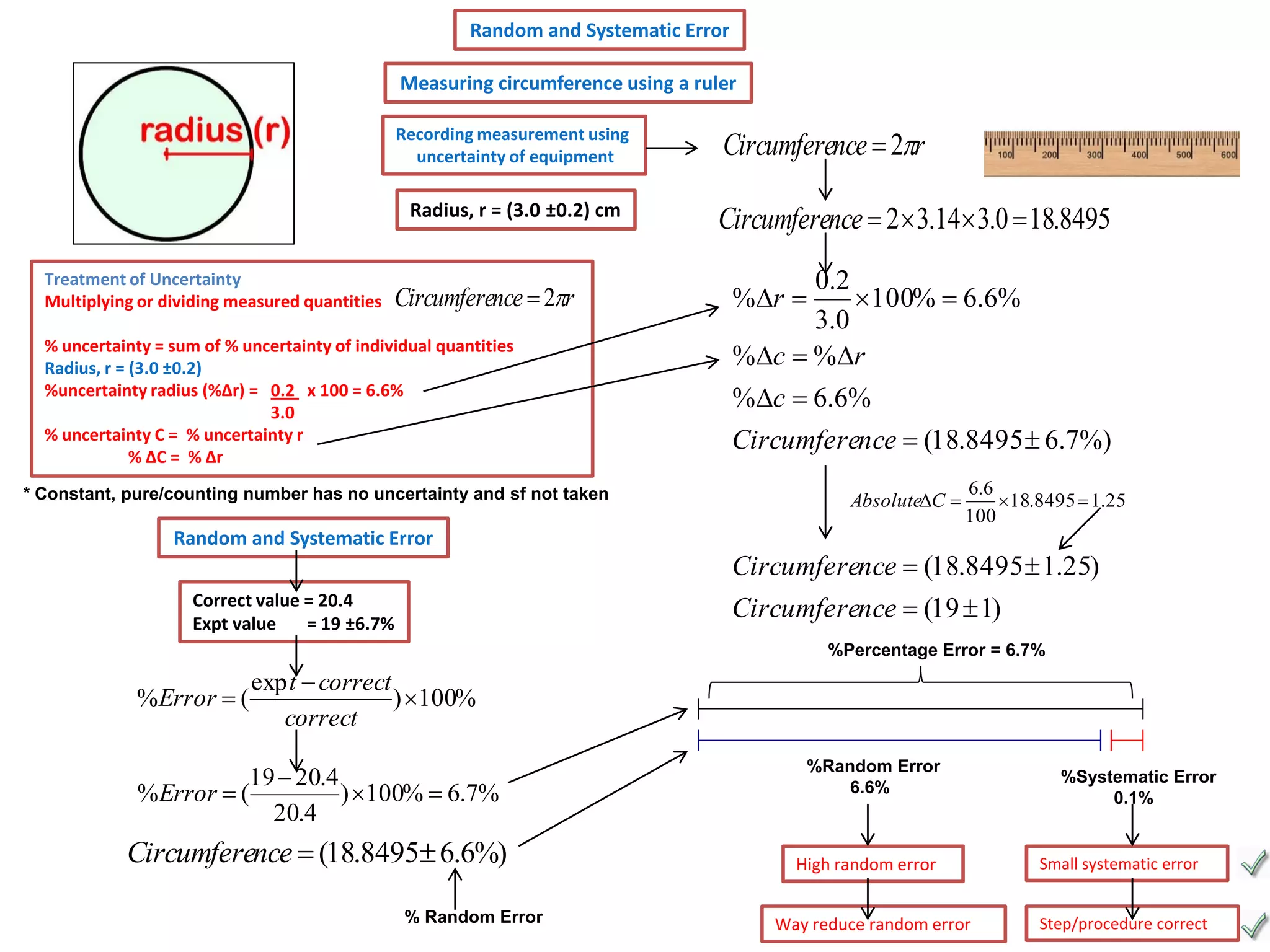
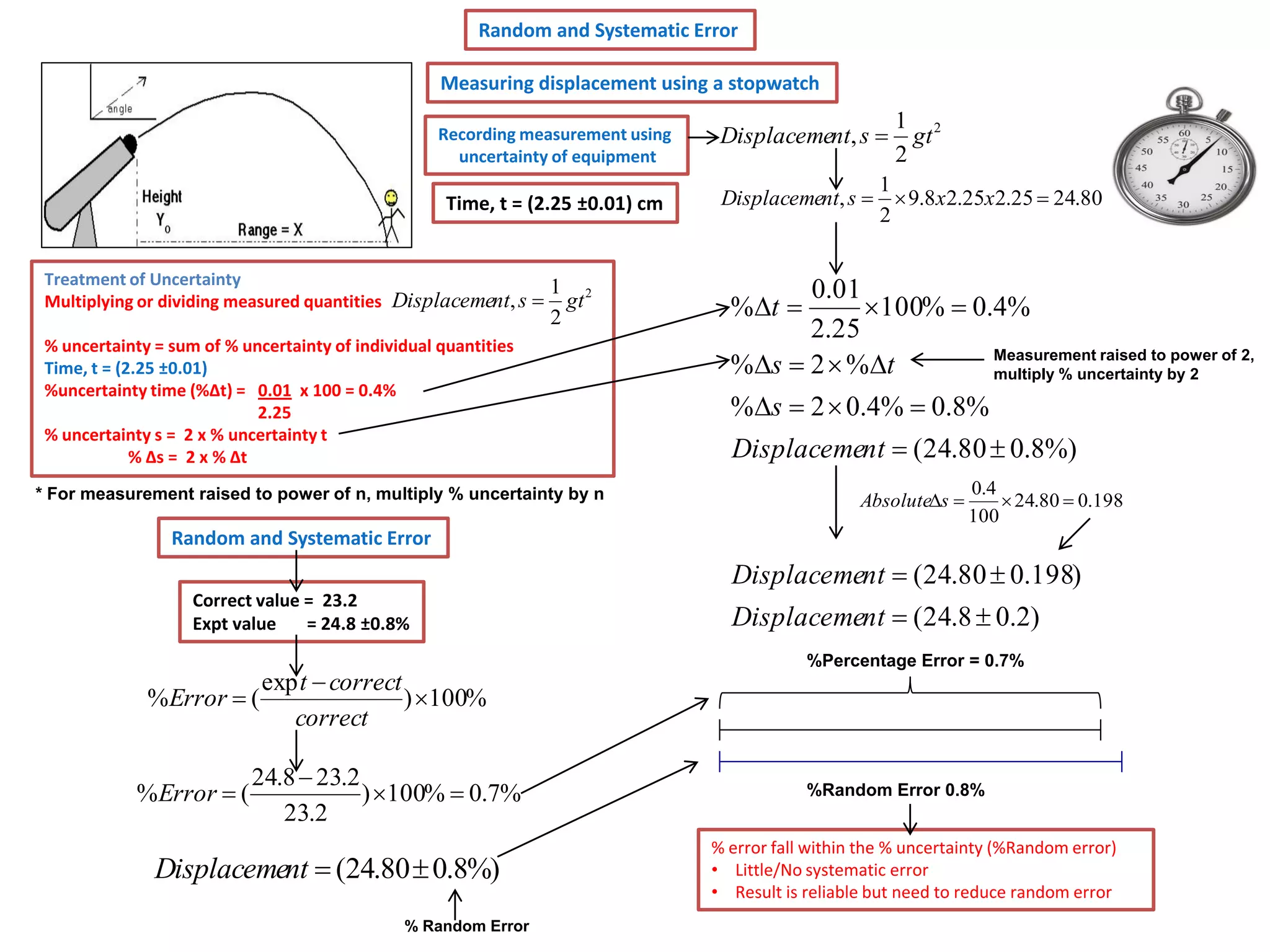
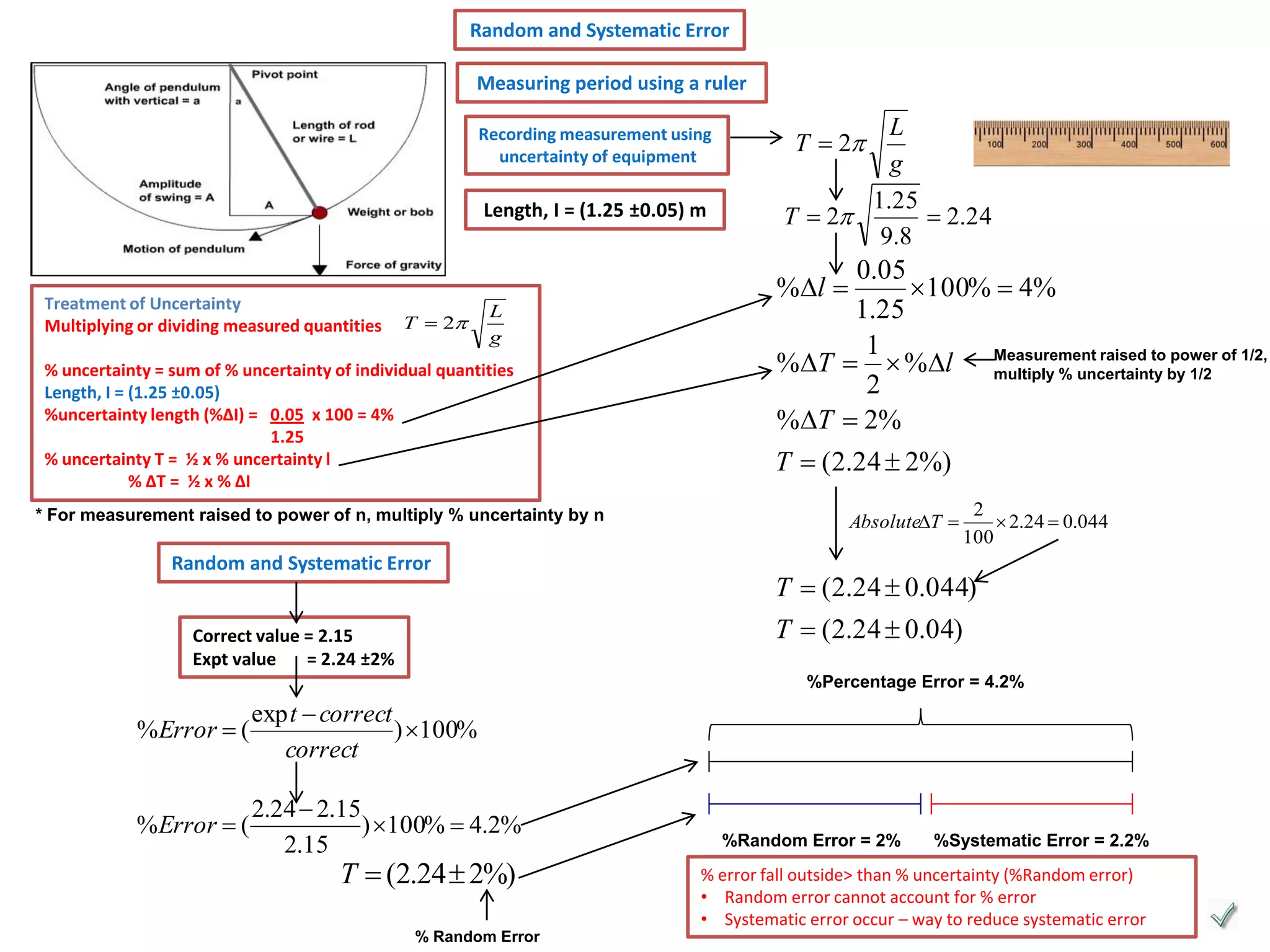
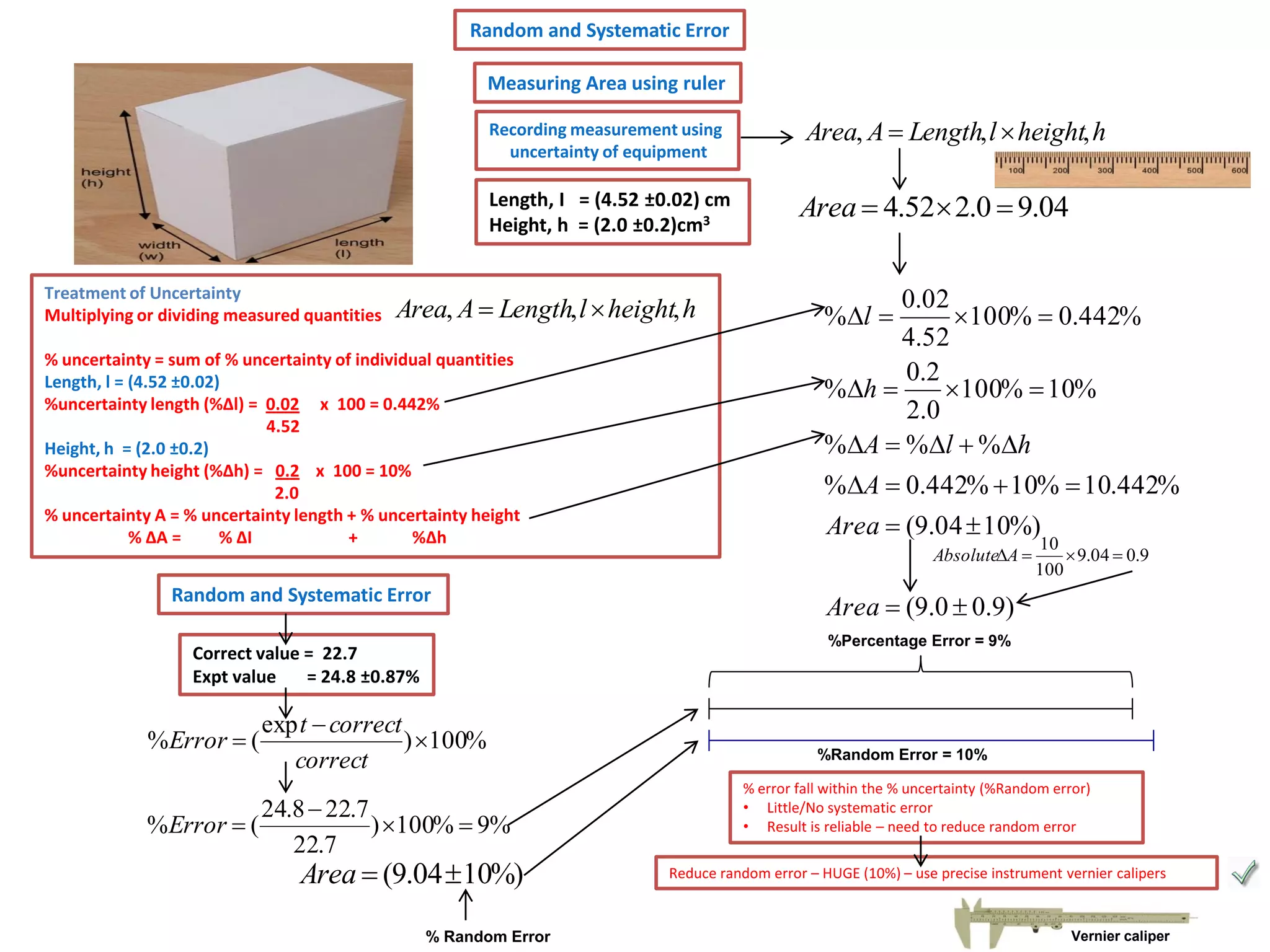
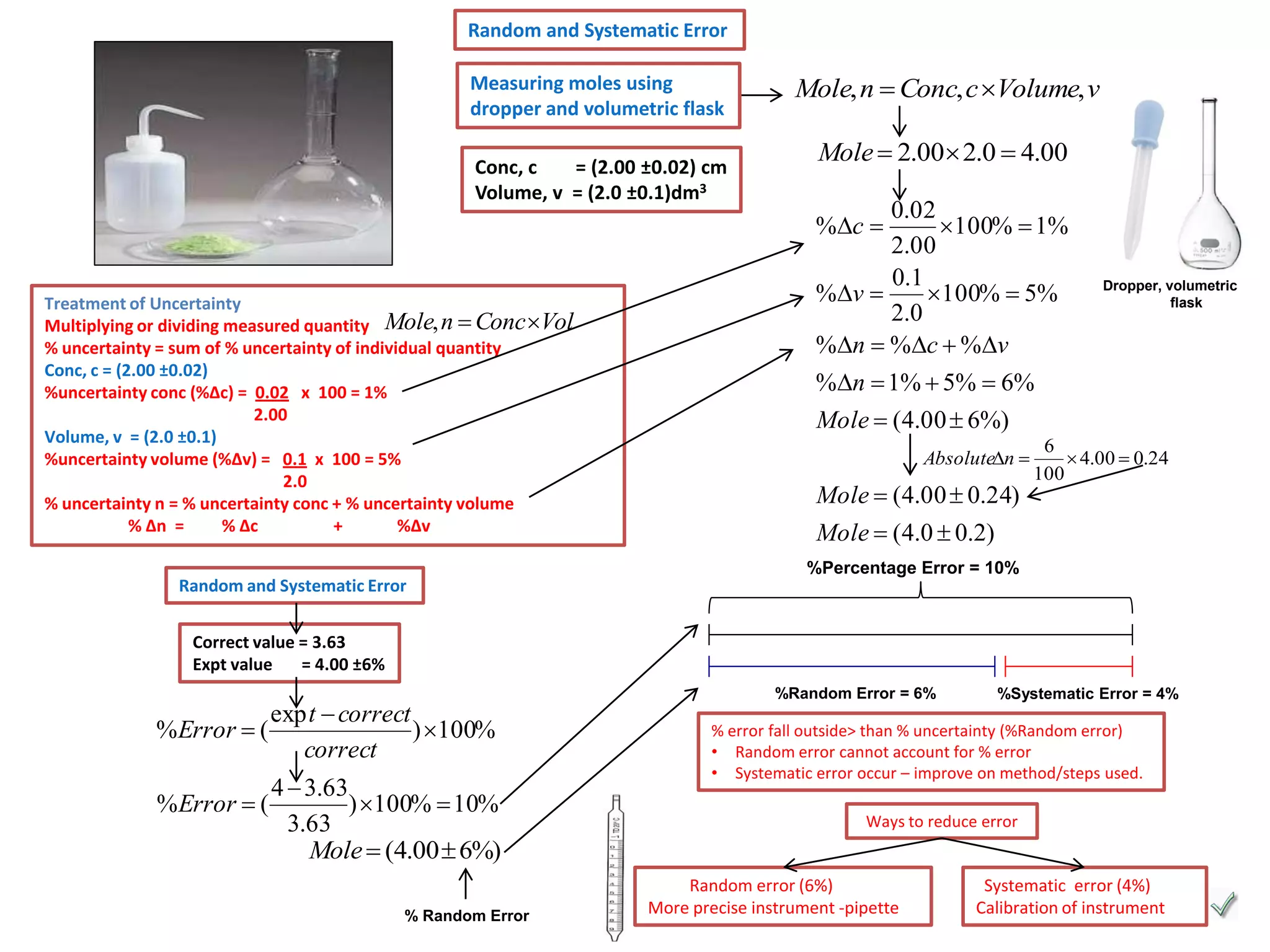
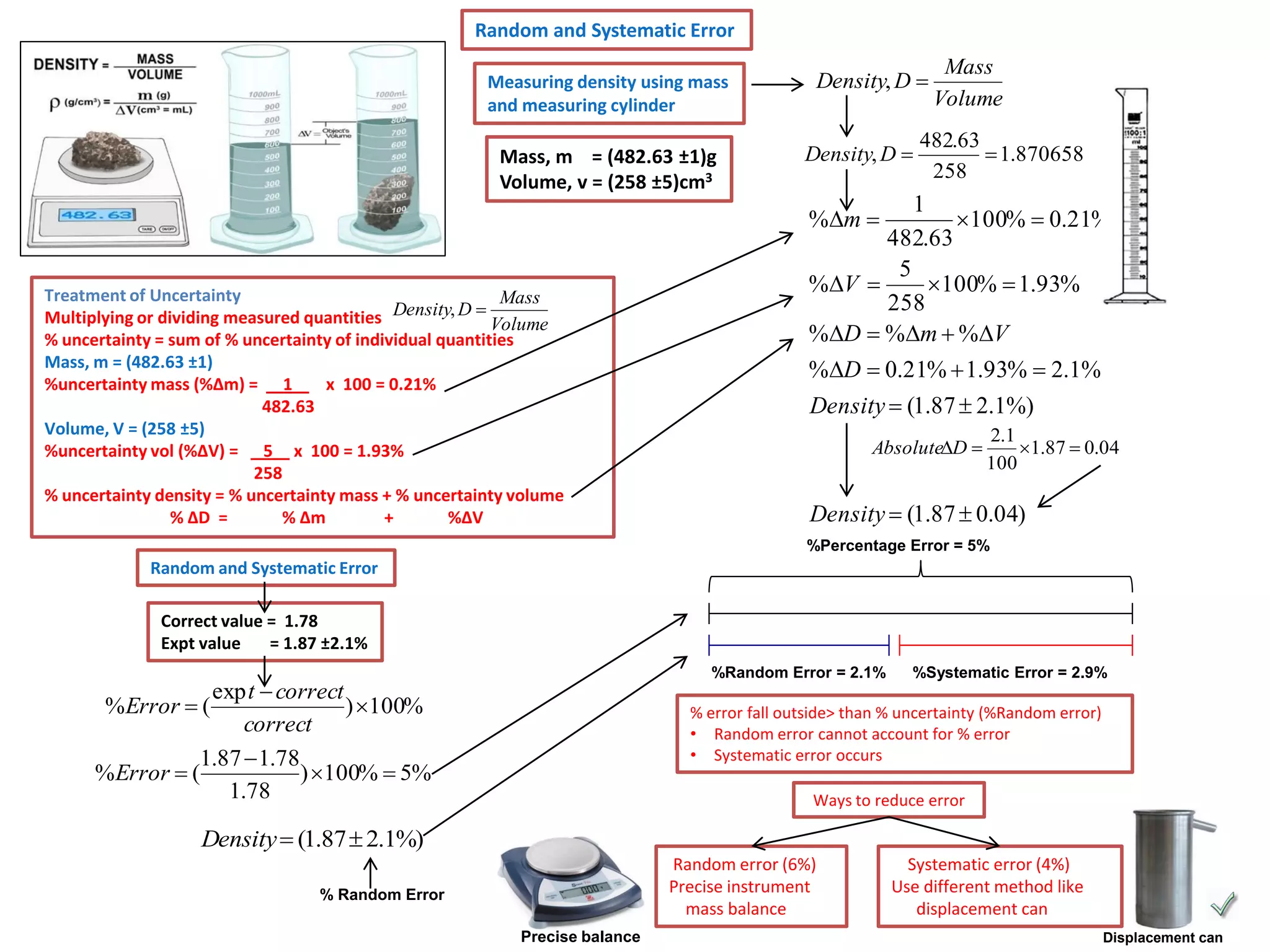
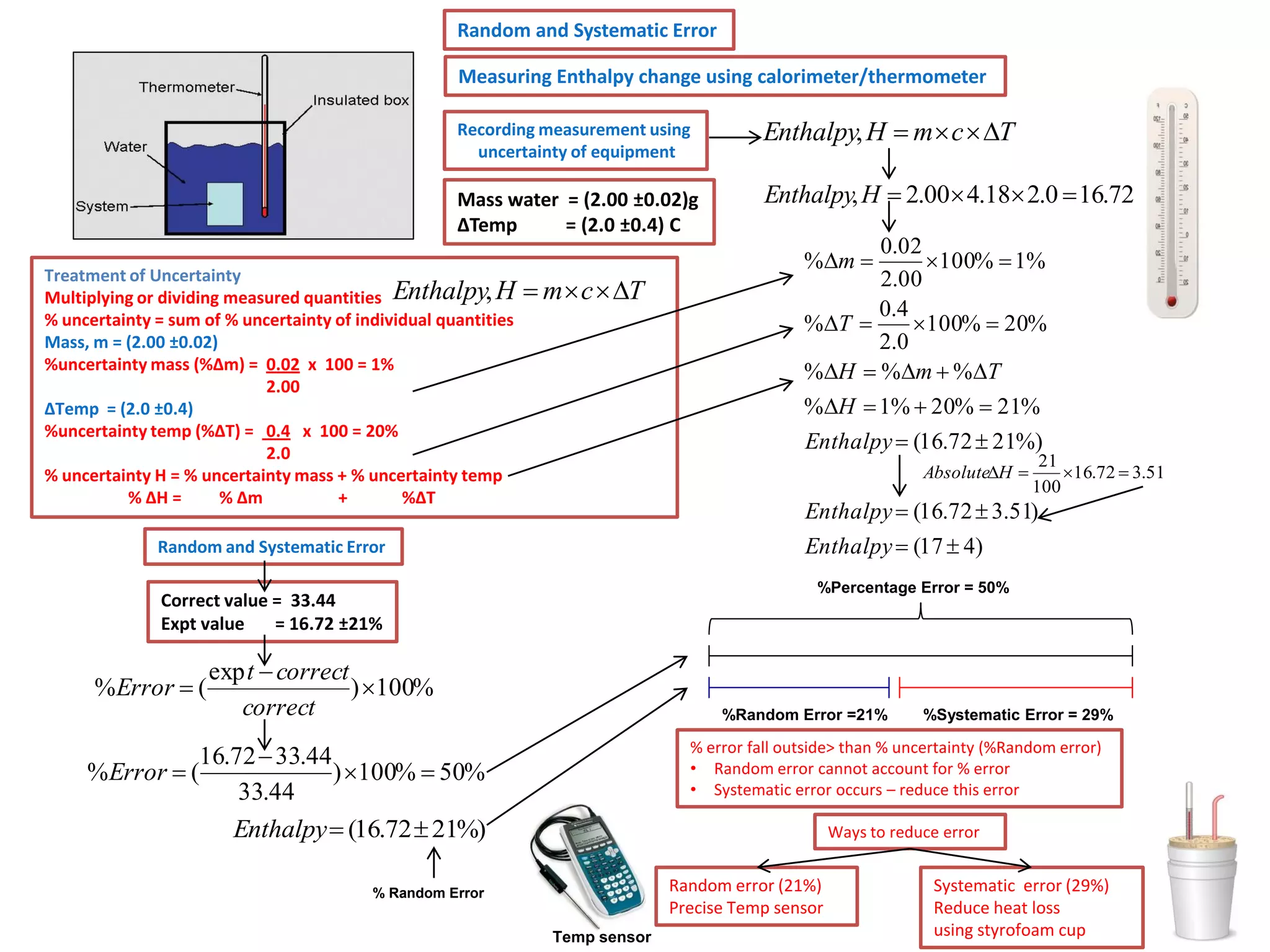
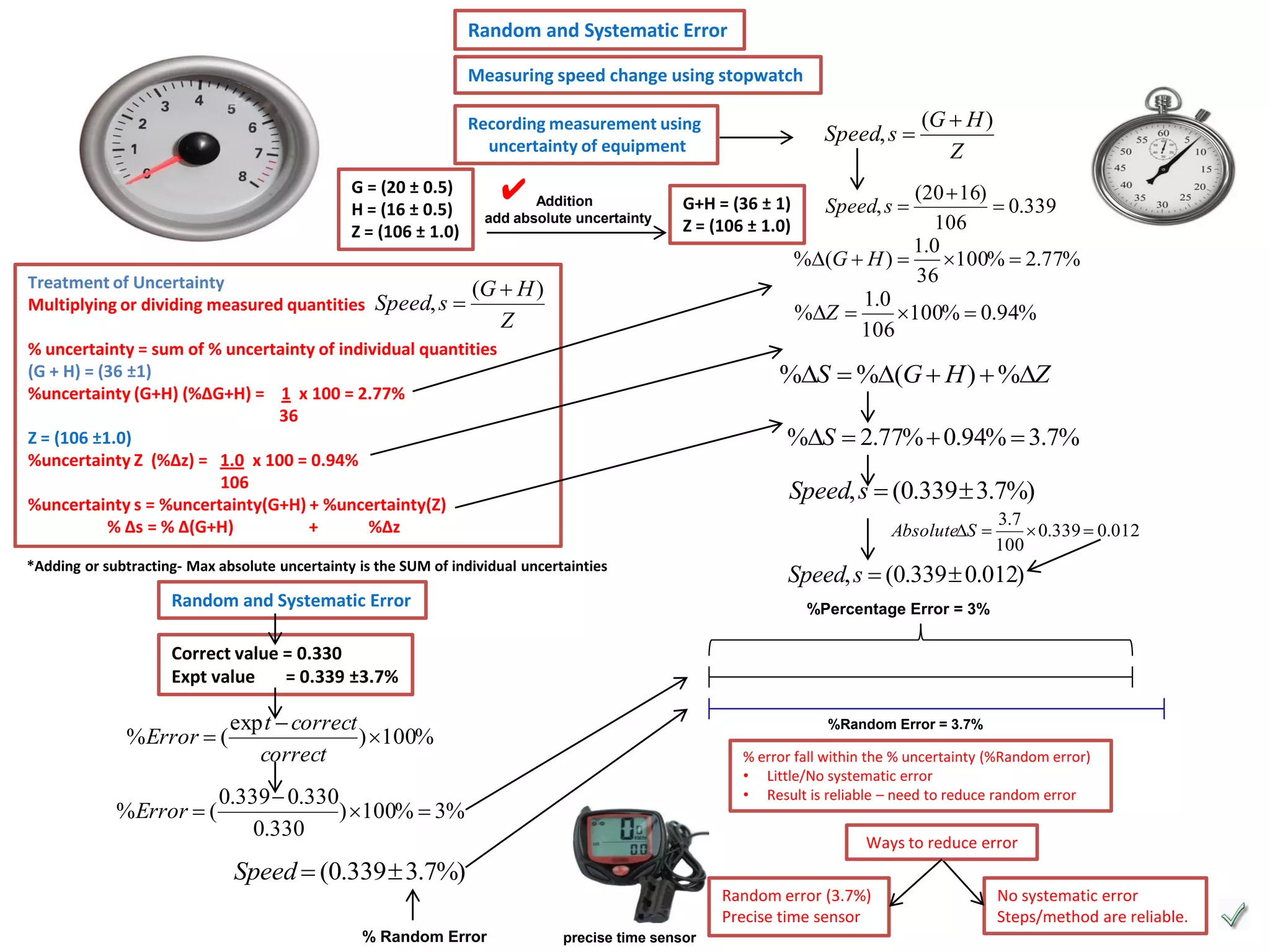
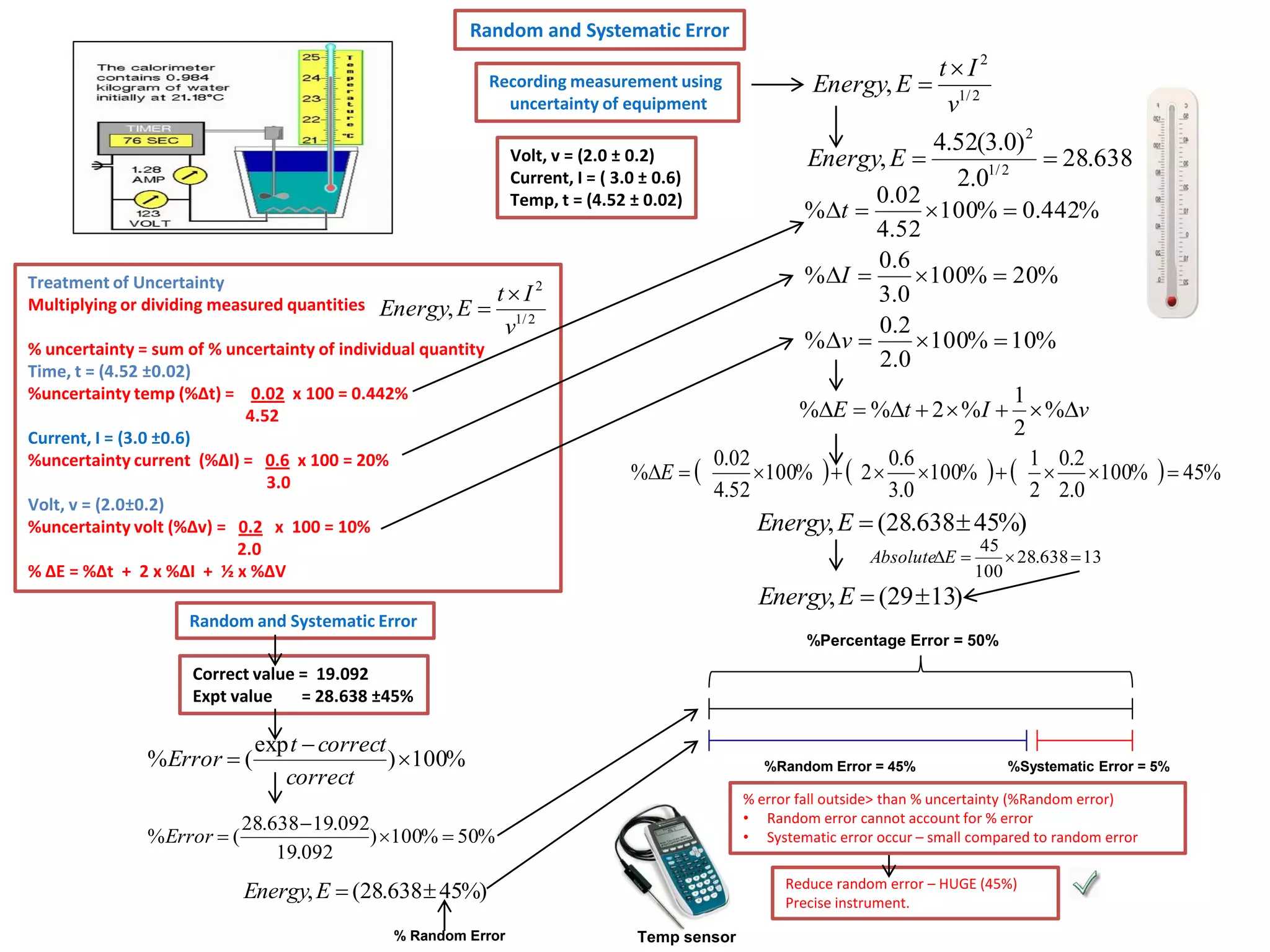
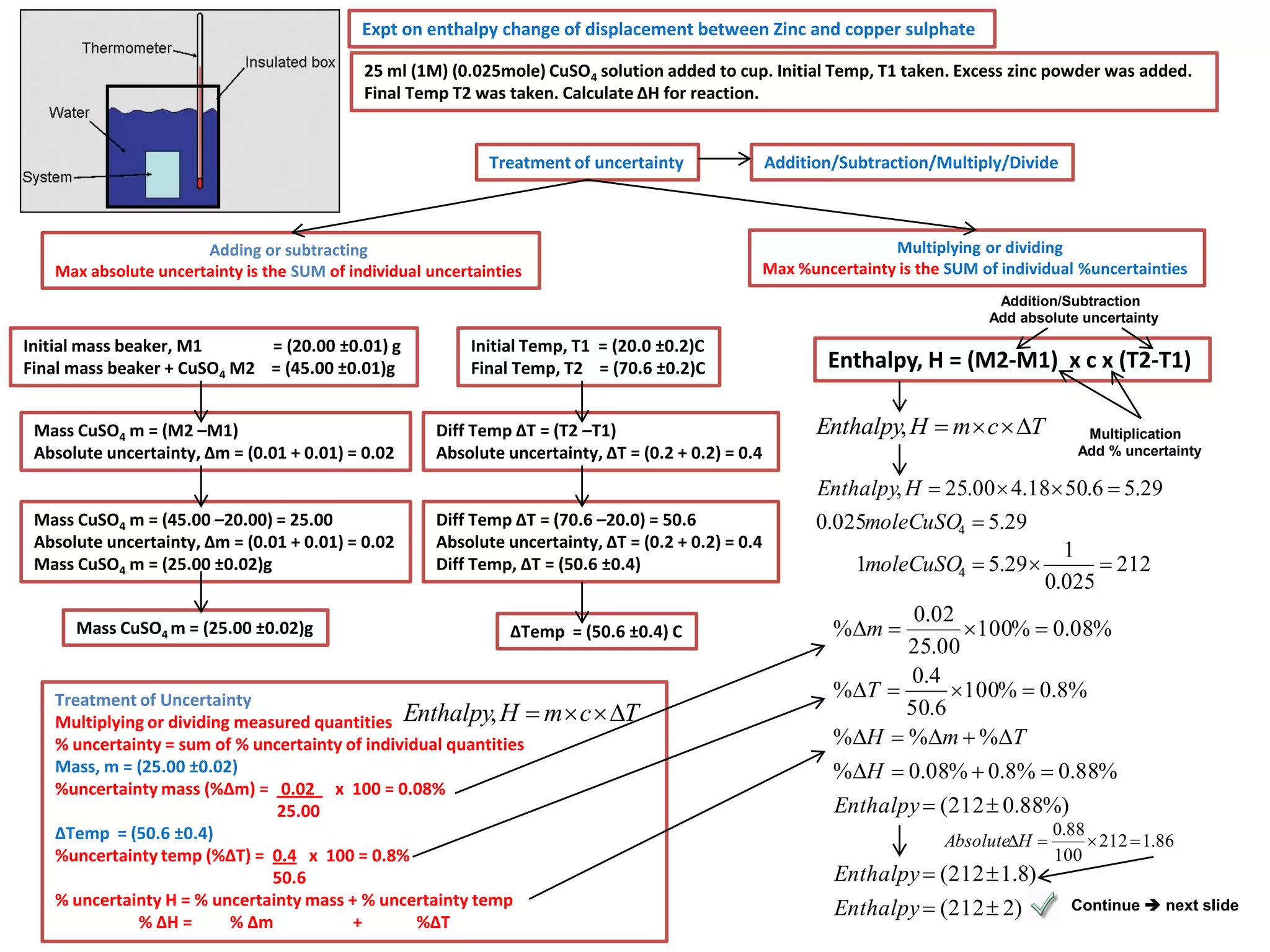
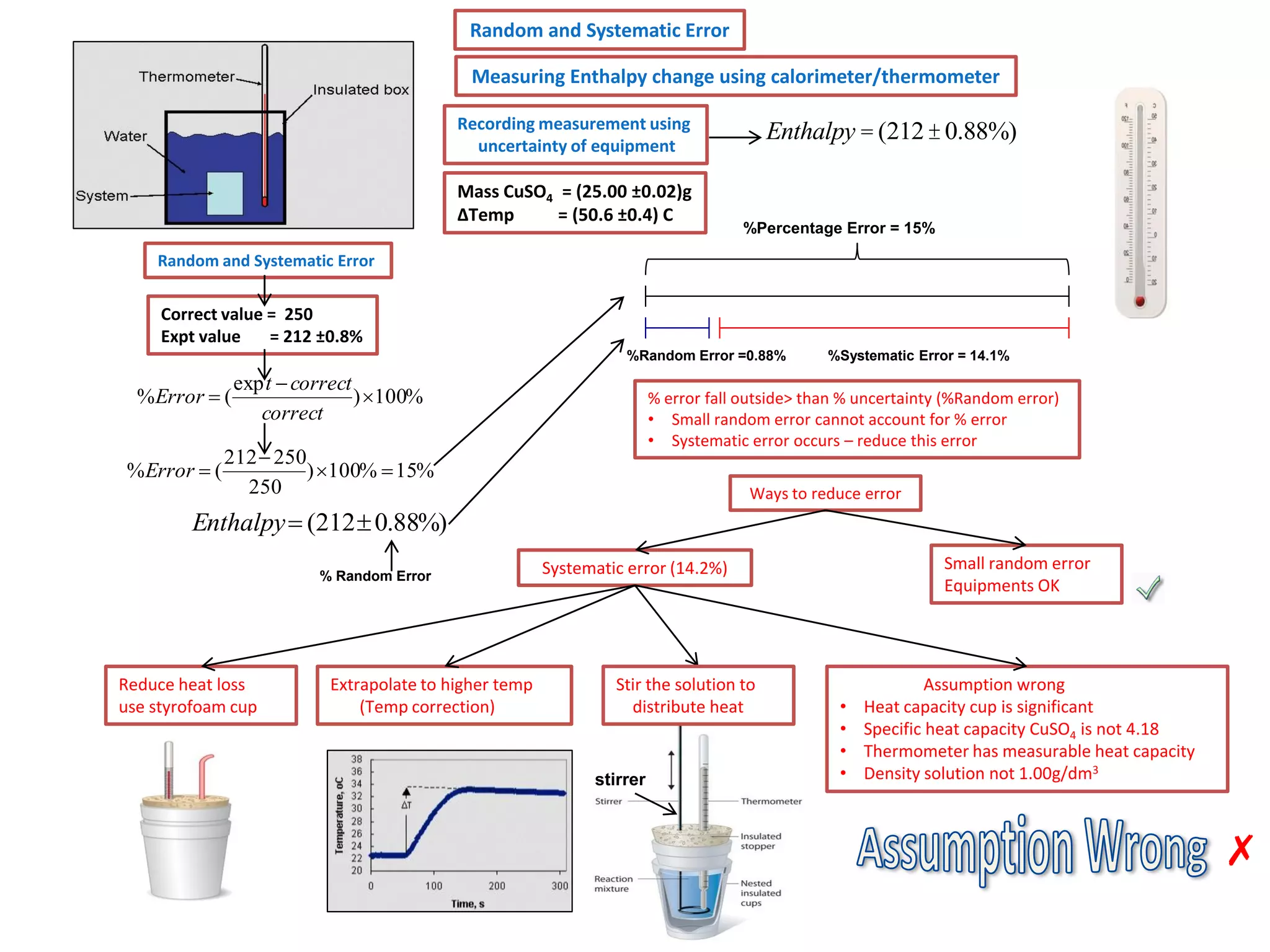
Every measurement has an associated error that affects its precision and accuracy. There are two types of errors - random error and systematic error. Random error affects precision while systematic error affects accuracy. Precision refers to the closeness of repeated measurements while accuracy refers to how close the measurement is to the true value. The percentage uncertainty of a measurement is calculated as the sum of the percentage uncertainties of the individual quantities involved. Measurements with uncertainties that account for the total percentage error are considered reliable while those with uncertainties that do not account for the total percentage error may have unidentified systematic errors. Reducing random errors involves improving measurement techniques while reducing systematic errors involves improving equipment calibration and measurement methods.