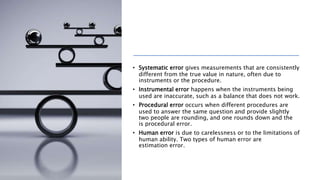
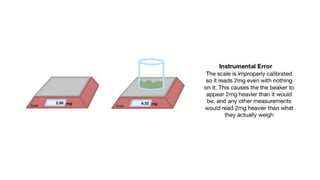
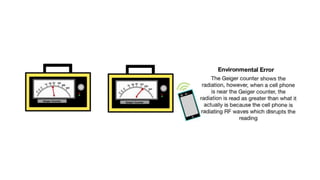
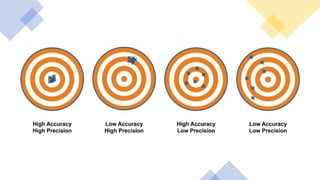
The document explains two types of errors in experimental physics: random errors, caused by chance and variability in measurements, and systematic errors, which consistently deviate from the true value, often due to inaccurate instruments or procedures. It outlines how to determine measurement uncertainty, emphasizing the importance of writing measurements in the format 'value ± uncertainty,' and describes how to calculate absolute and percentage uncertainties for addition, subtraction, multiplication, and division. Furthermore, the text defines accuracy and precision in measurements and provides examples to illustrate different relationships between measured values and true values.