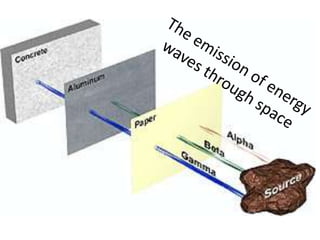
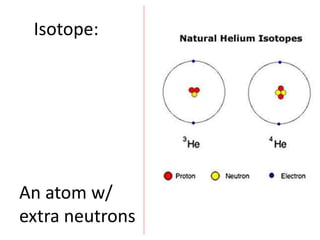
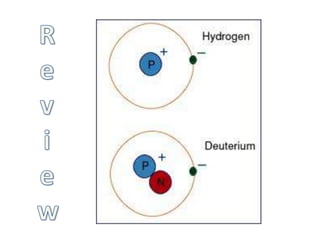
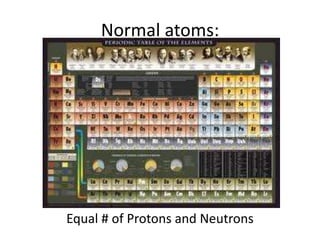
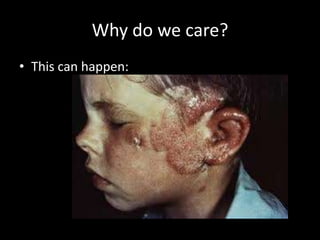
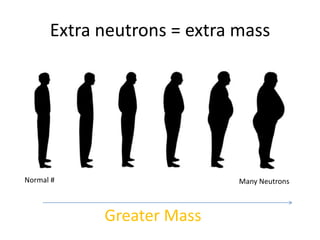
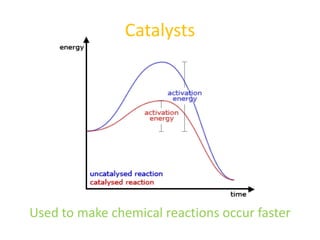
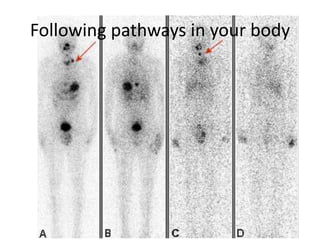
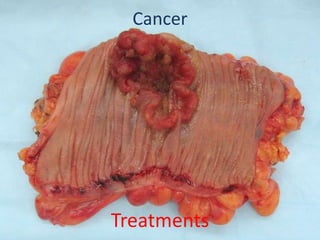
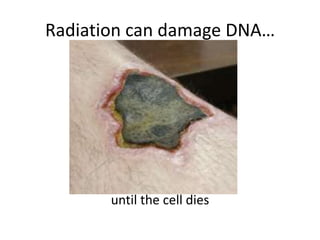
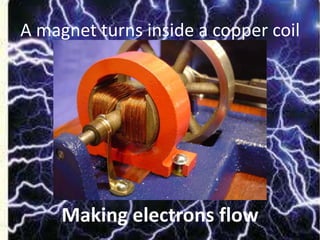
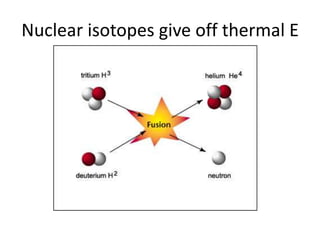
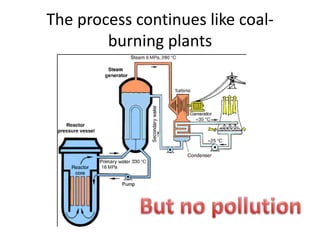
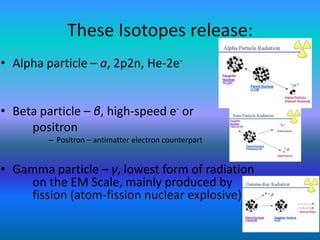
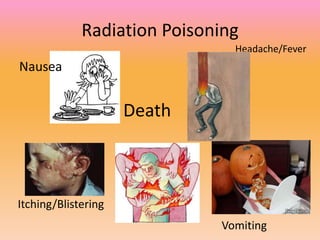
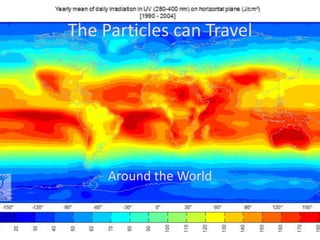
The document explains nuclear radiation, describing it as energy waves emitted from radioisotopes and the significance of isotopes in various applications, including cancer treatment and energy production. It outlines the functioning of nuclear power plants compared to coal-burning plants, highlighting the benefits of nuclear energy despite potential risks and disasters like Chernobyl and Fukushima. Additionally, it discusses the types of radiation and their effects on health, concluding with a note on how to protect oneself from radiation exposure.