This document discusses the three main types of radioactive emissions: alpha particles, beta particles, and gamma radiation.
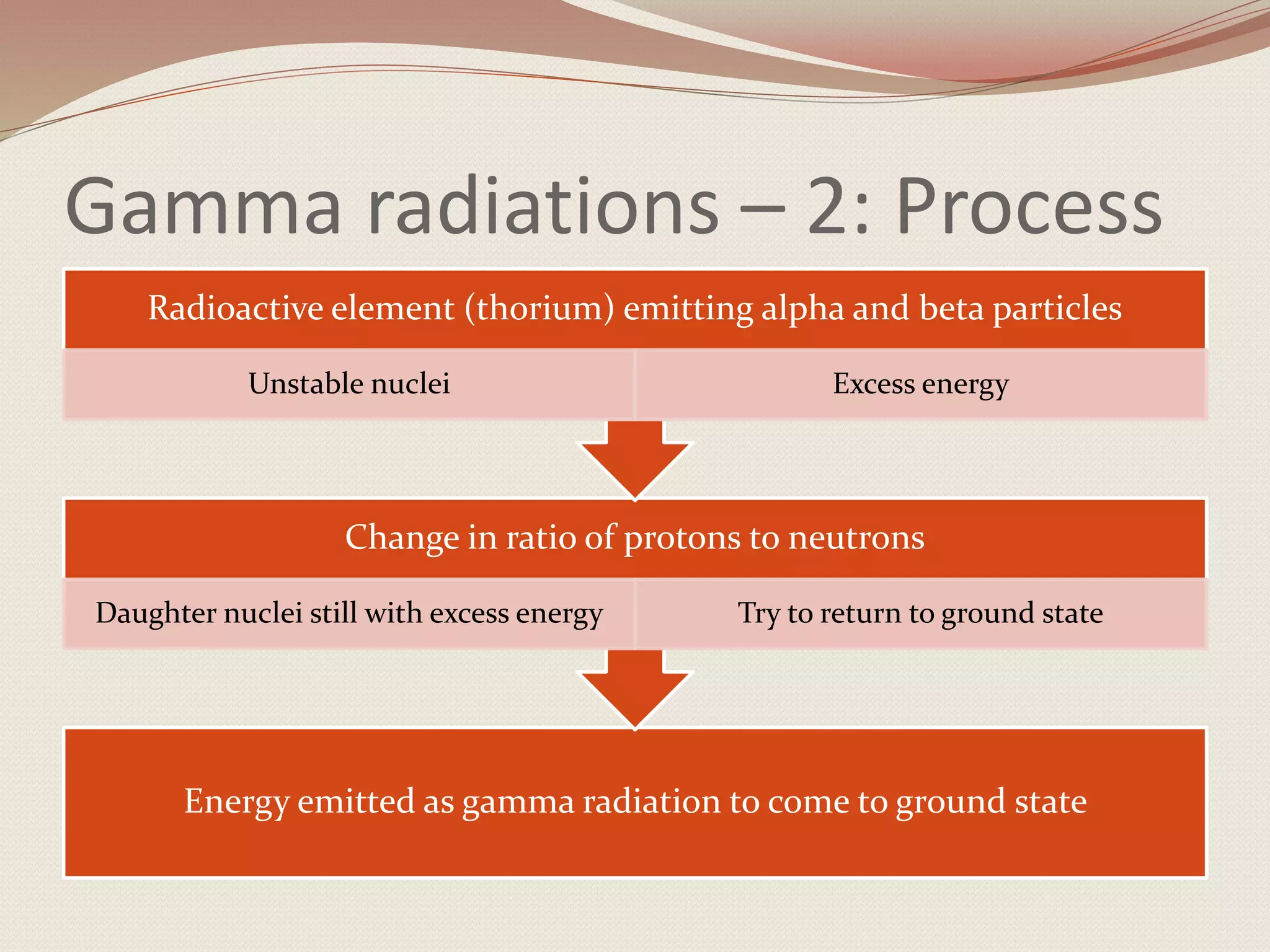
Alpha particles are made up of two protons and two neutrons and are the least penetrating emission. Beta particles can be emitted as electrons or positrons. Gamma radiation involves the emission of electromagnetic radiation from an excited nucleus as it transitions to the ground state.
The document explains the key properties and effects of each type of emission, and how they result in the radioactive decay and transmutation of elements in a decay series until a stable nuclide is reached.