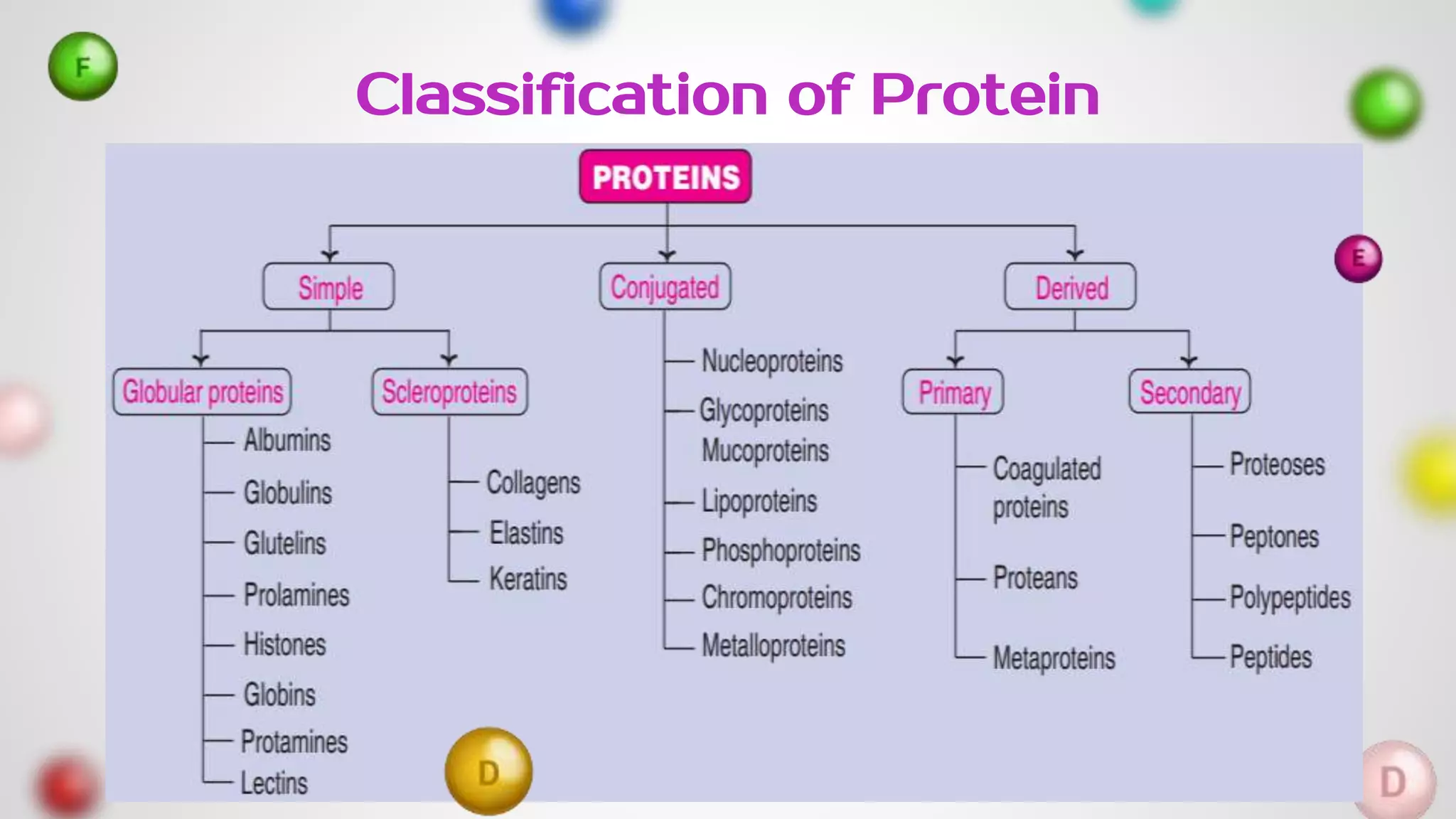
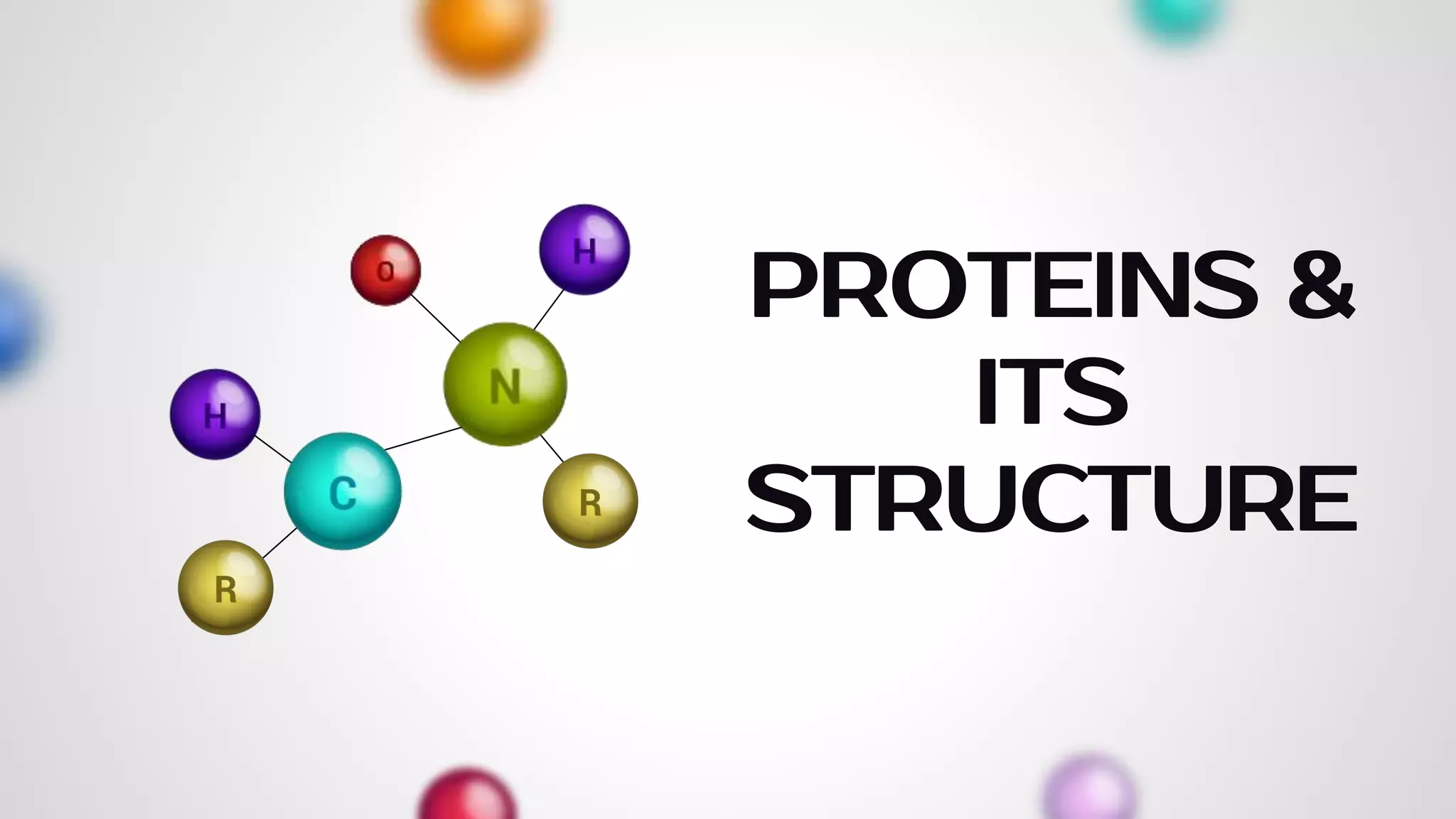
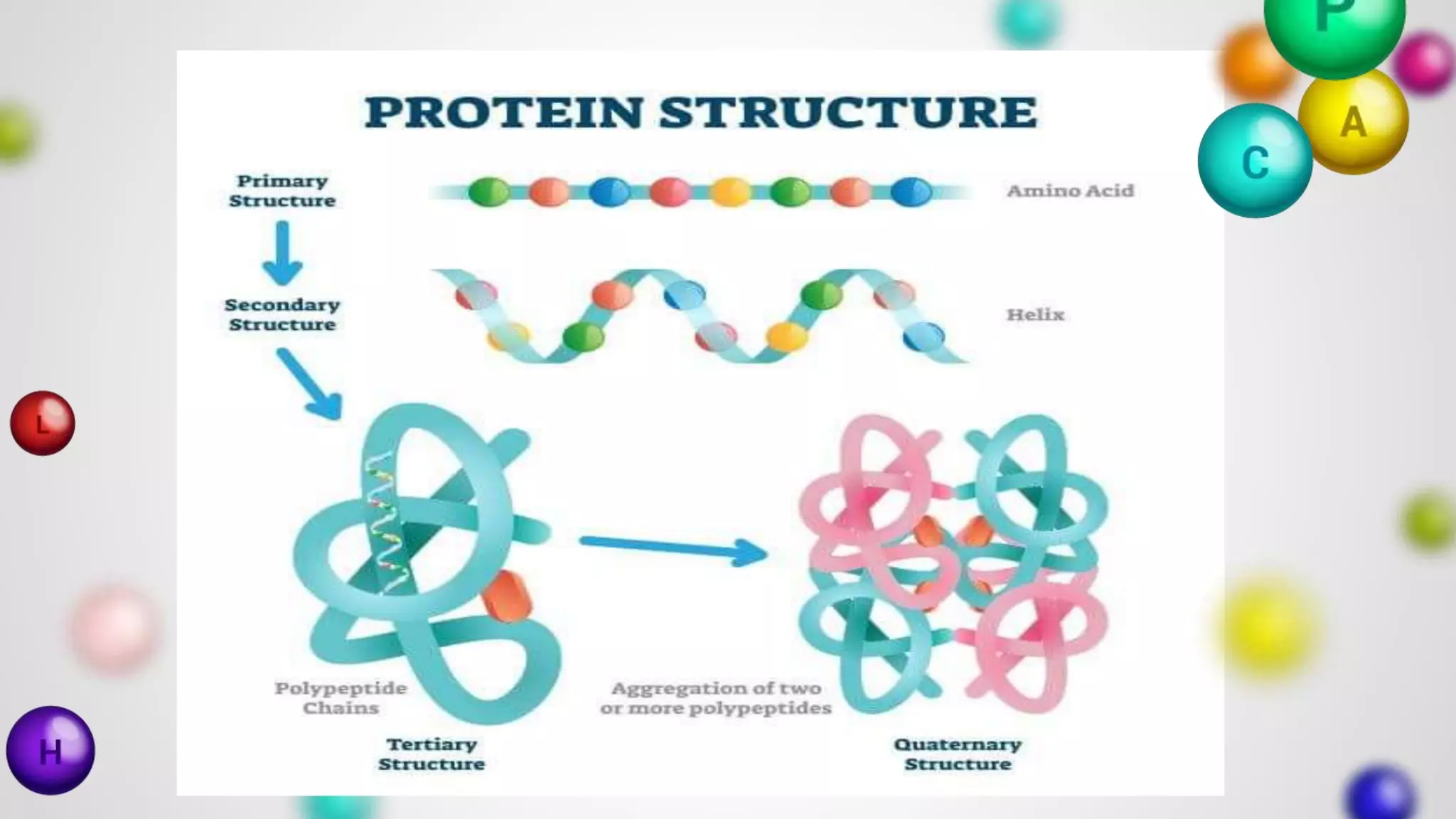
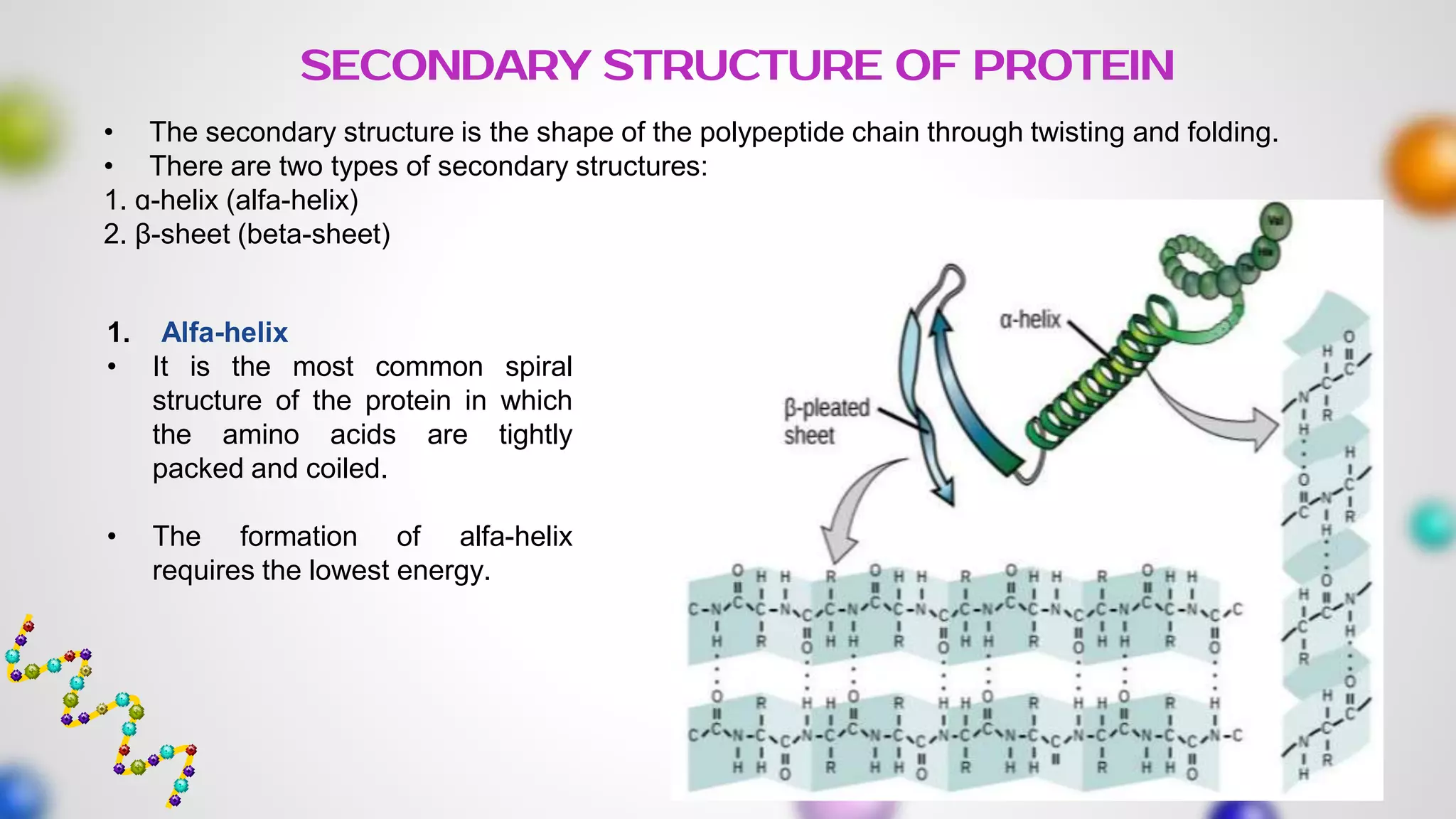
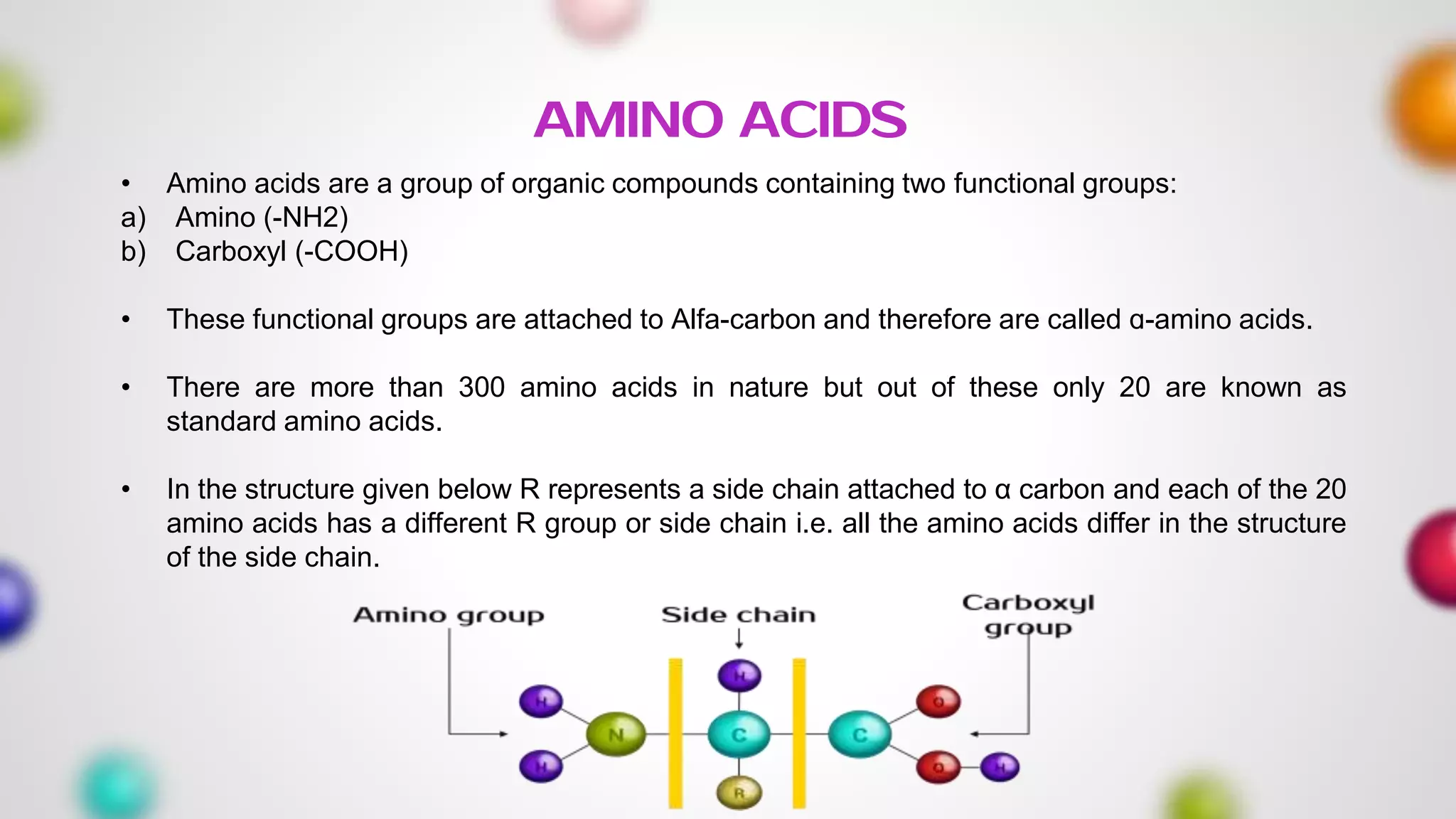
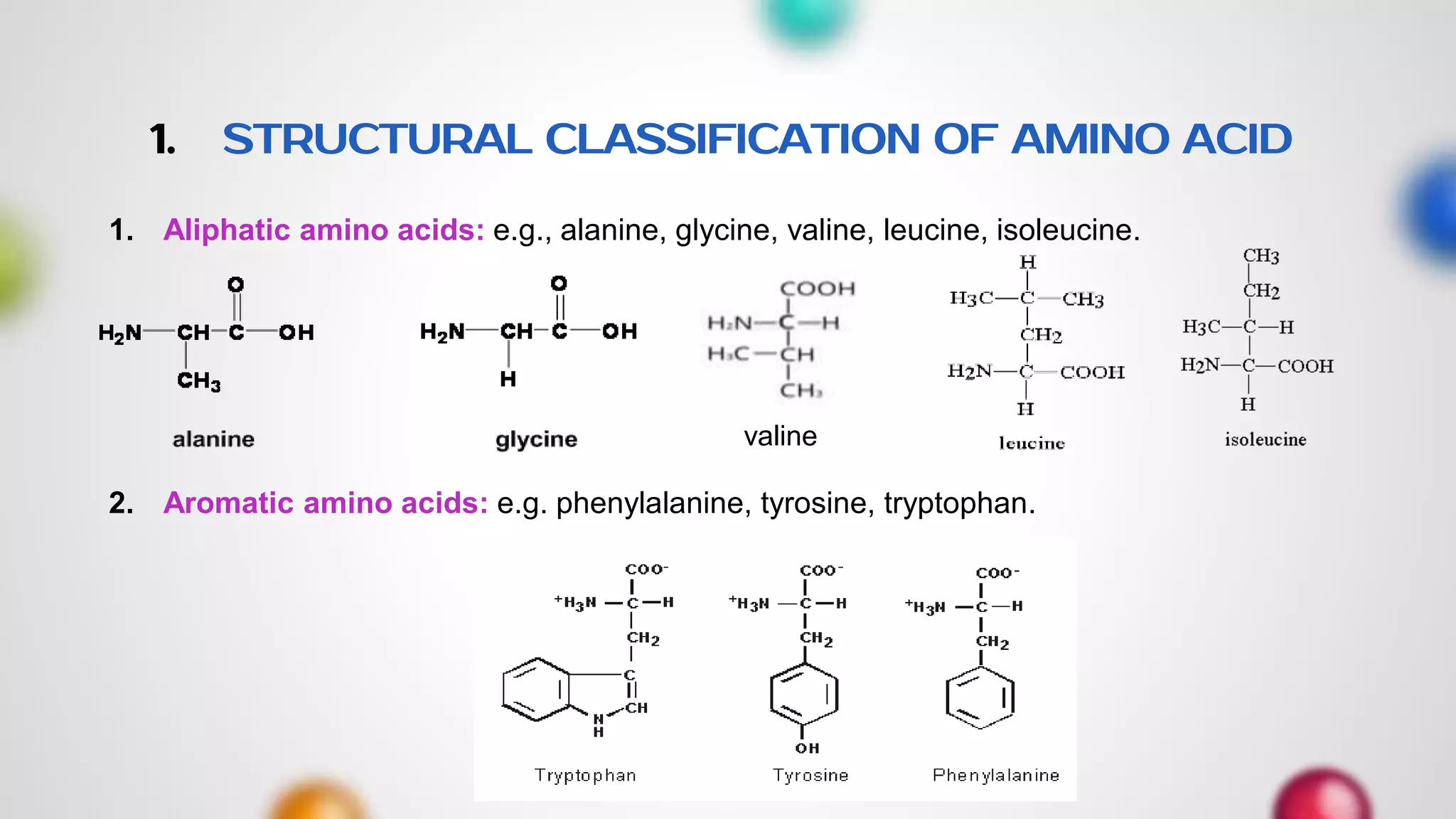
The document provides an extensive overview of proteins, covering their structures, functions, classifications, and the role of amino acids in protein synthesis. It explains the importance of proteins in bodily functions, their classification into simple, conjugated, and derived proteins, and highlights the consequences of protein malnutrition such as kwashiorkor and marasmus. Various tests for protein identification, as well as the properties of amino acids, are also discussed.