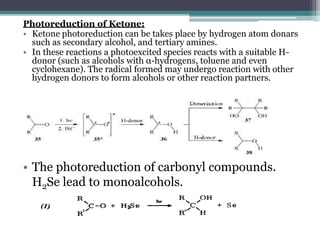
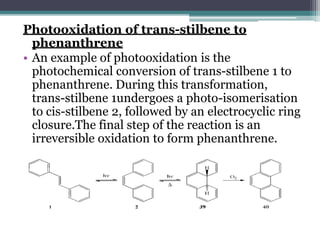
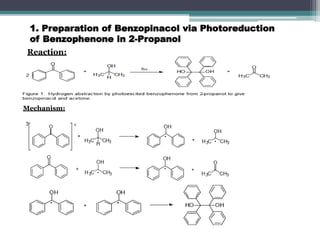
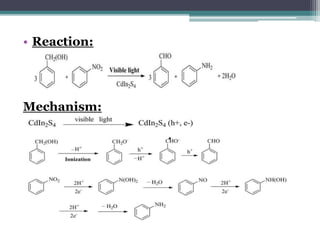
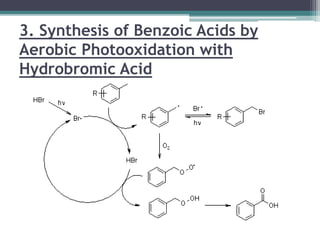
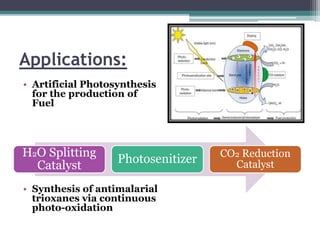
The document discusses various photooxidation and photoreduction reactions in organic synthesis. It begins by introducing photochemistry and defining related terms. It then provides examples of photoreduction of ketones and aromatic hydrocarbons. Examples of photooxidation reactions include the conversion of trans-stilbene to phenanthrene and the synthesis of benzoic acids via aerobic photooxidation. The document also describes the mechanism and advantages of using a CdIn2S4 photocatalyst for selective photosynthesis of organic aromatic compounds under visible light.