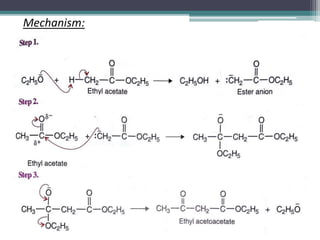
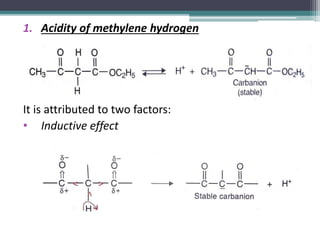
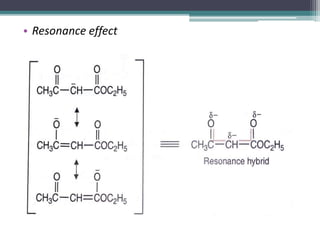
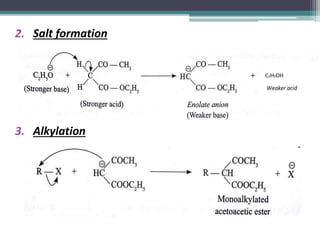
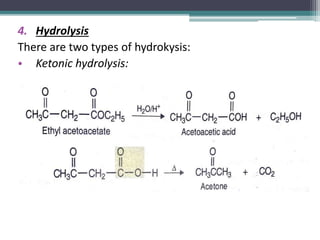
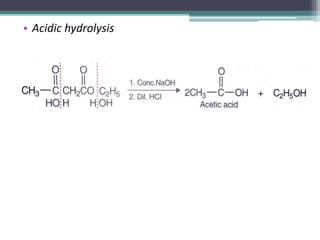
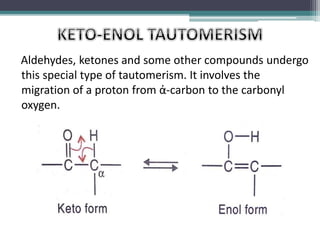
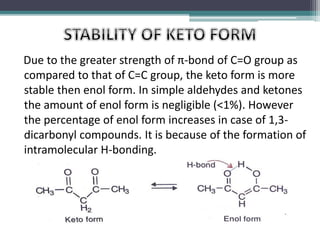
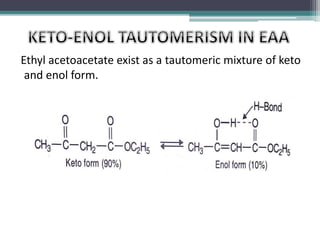
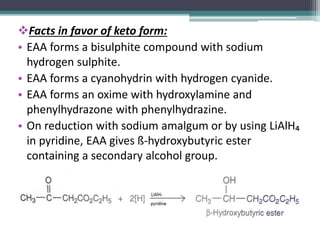
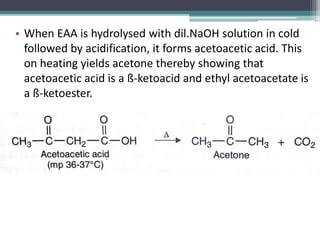
This document presents information on ethyl acetoacetate (EAA), including its preparation via Claisen condensation, physical and chemical properties, keto-enol tautomerism, and synthetic uses. EAA is a colorless, pleasant smelling liquid that is sparingly soluble in water but freely soluble in organic solvents. As the methylene hydrogen is acidic, EAA can be used to synthesize many compounds through reactions like salt formation, alkylation, and hydrolysis. EAA exists as a mixture of the keto and enol forms, with evidence provided to support both. It is commonly used to synthesize carboxylic acids, α,β-unsaturated acids, ketones,