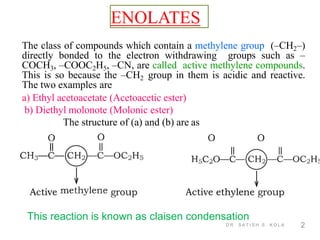
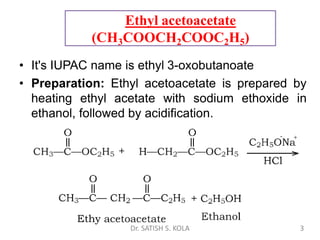
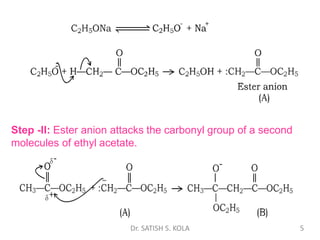
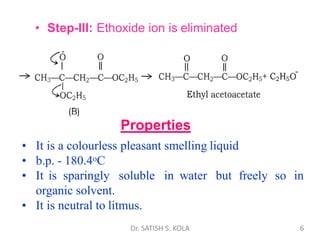
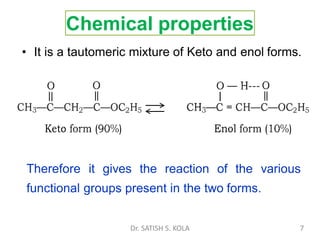
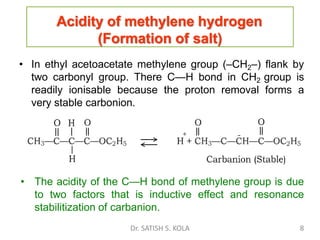
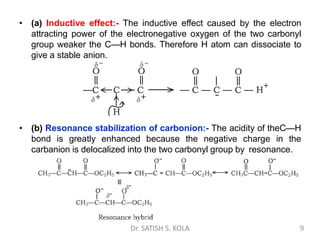
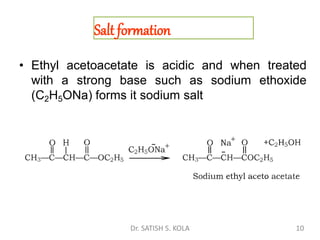
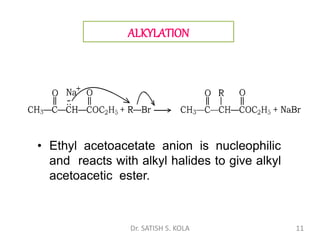
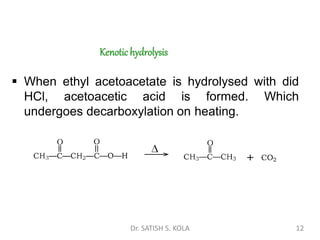
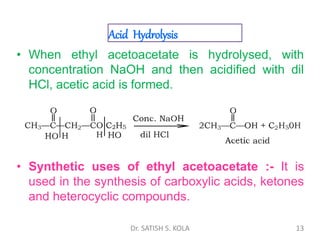
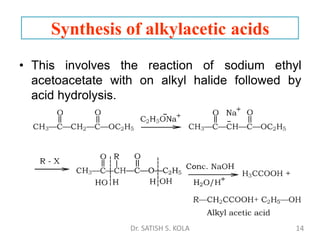
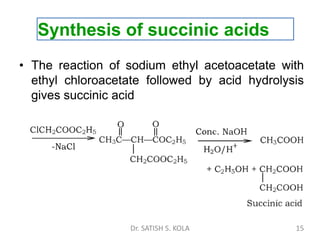
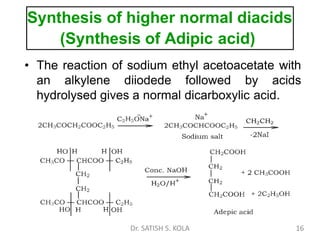
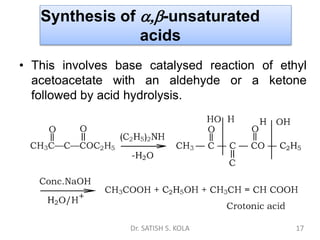
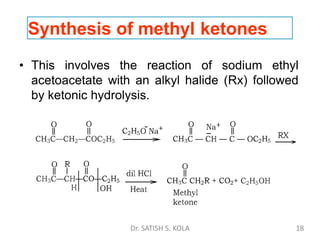
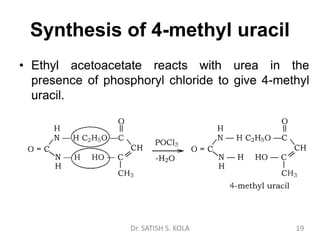
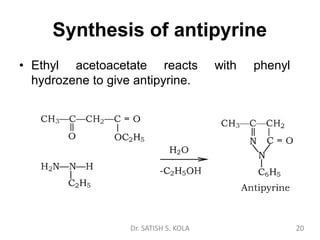
Dr. Satish S. Kola discusses organic synthesis via enolates, specifically focusing on ethyl acetoacetate. Ethyl acetoacetate is prepared through the heating of ethyl acetate with sodium ethoxide followed by acidification. It undergoes Claisen condensation, forming an alcohol and β-keto ester. Ethyl acetoacetate displays properties of both the keto and enol forms. It is acidic, forming salts when treated with bases through the ionization of the methylene hydrogen. Its acidity is enhanced by inductive effects and resonance stabilization of the carbanion. Ethyl acetoacetate is used synthetically to produce carboxylic acids, ket