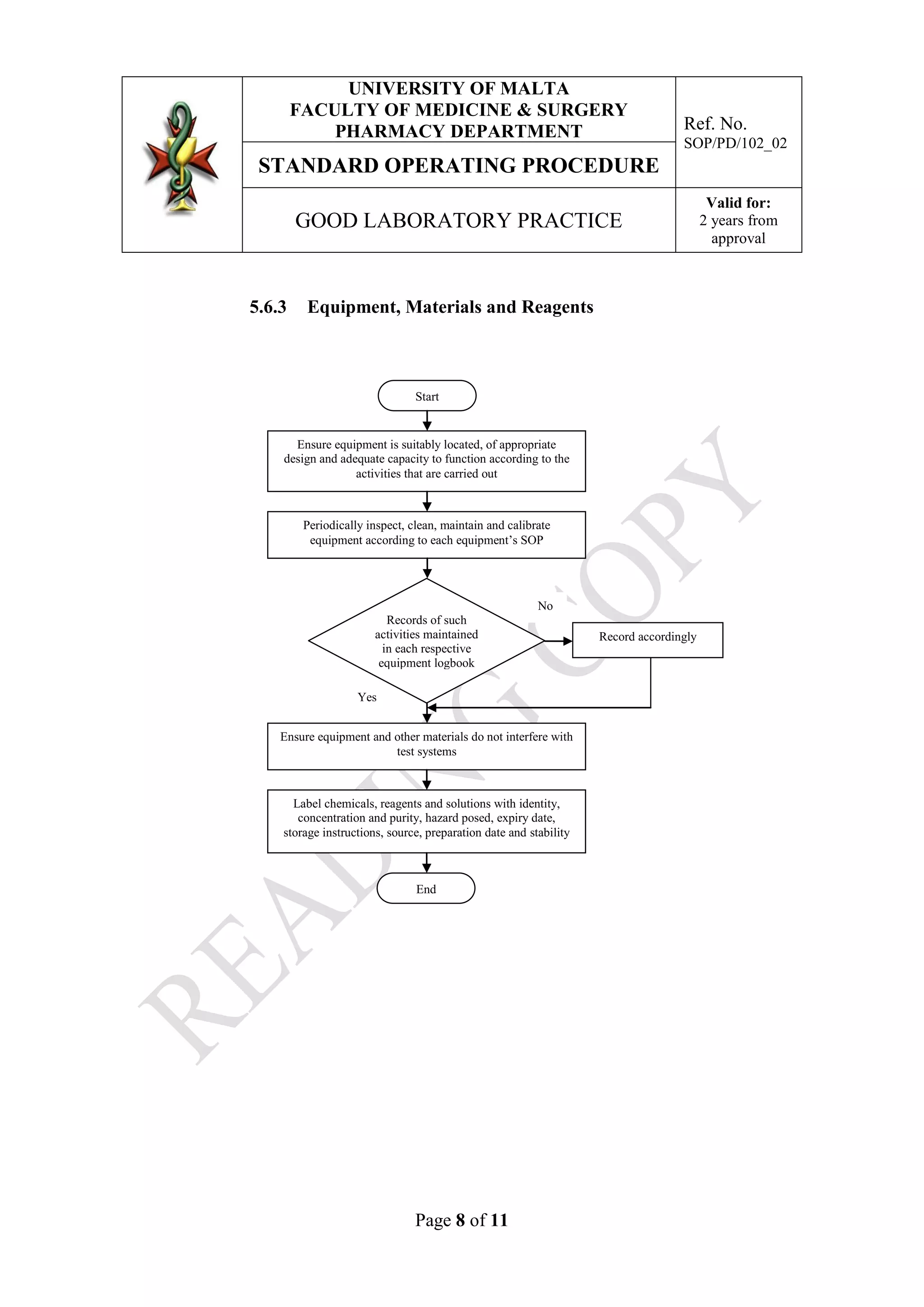
This document outlines the Good Laboratory Practice (GLP) guidelines for laboratories at the University of Malta's Pharmacy Department. It details responsibilities and procedures for facilities, personnel, equipment, materials, reagents, test substances and standard operating procedures. The guidelines are meant to ensure quality practices are followed to plan, perform, monitor and report activities conducted in the laboratories. Responsible staff must implement the procedures around facilities management, training qualified personnel, properly handling equipment and supplies, and following approved standard operating procedures.