This document discusses manufacturing operations and controls to prevent mix-ups and cross contamination. It outlines precautions like proper air handling, segregated areas, and status labeling. Processing of intermediates and bulk products must be documented and checks put in place to ensure quality like verifying identity and yields. Calibration records and batch production and control records are required. Contamination can occur from materials, areas, equipment or people so trained personnel and technical measures like separate production areas are important controls.
![PRESENTED BY :
S A C H I N PAWA R M P H A R M F. Y. [ Q A ]
GUIDED BY:
R . S . S A K H A R E S I R
S C H O O L O F P H A R M A C Y S RT M U N N A N D E D .
MANUFACTURING
OPERATION AND CONTROLS](https://image.slidesharecdn.com/manufacturingoperationandcontrolssachin-171101040315/85/Manufacturing-operation-and-controls-1-320.jpg)



![SANITATION IN THE MANUFACTURING
PREMISES :
1] The manufacturing premises shall be cleaned and in an
orderly manner
2] The manufacturing areas shall not be used for other
operations
3 ]A routine sanitation program shall be drawn up which
shall be properly recorded and which indicate–
a) specific areas to be cleaned and cleaning intervals
b) cleaning procedure to be followed
c) personnel assigned to and responsible for the cleaning
operation.](https://image.slidesharecdn.com/manufacturingoperationandcontrolssachin-171101040315/85/Manufacturing-operation-and-controls-5-320.jpg)







![Controlling of mix ups and contamination
A] EXHAUST SYSTEM
Certain procedure should be used to control mix
ups and contamination
exhaust system with proper air filtration and
dust collection
there should be separate air handling unit
Their should be air pressure control maintained
Packaging unit should be well separated i.e
1.2-1.5 m in two adjacent packaging line](https://image.slidesharecdn.com/manufacturingoperationandcontrolssachin-171101040315/85/Manufacturing-operation-and-controls-13-320.jpg)
![B] TRAINED PEOPLE:
In pharma processing the people should be trained in
their job and also in their principle of CGMP
It should having discipline procedure of correct
procedure ,products training and equipment handling](https://image.slidesharecdn.com/manufacturingoperationandcontrolssachin-171101040315/85/Manufacturing-operation-and-controls-14-320.jpg)
![C] TEACHNICAL MEASURE
1. Production in segregated area
2. Provide appropriate air loss and system
3. Always use the cleaning equipment
4. Using a close system for material handling
and production](https://image.slidesharecdn.com/manufacturingoperationandcontrolssachin-171101040315/85/Manufacturing-operation-and-controls-15-320.jpg)


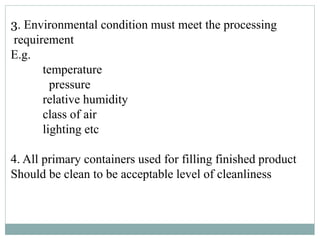




![DOCUMENTS REQUIRED
1] B.P.C.R for each batch produced
2] calibration records](https://image.slidesharecdn.com/manufacturingoperationandcontrolssachin-171101040315/85/Manufacturing-operation-and-controls-23-320.jpg)


