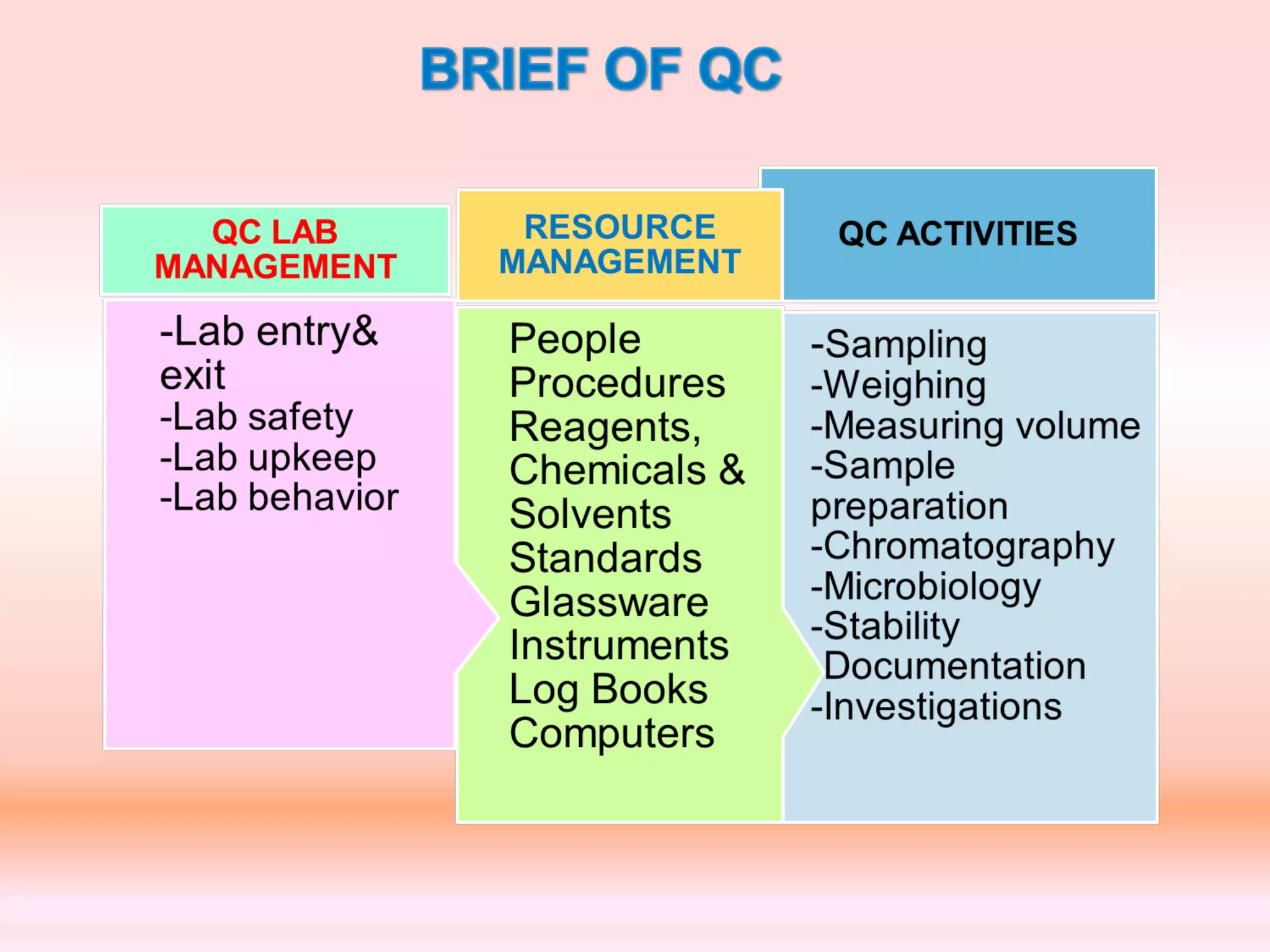
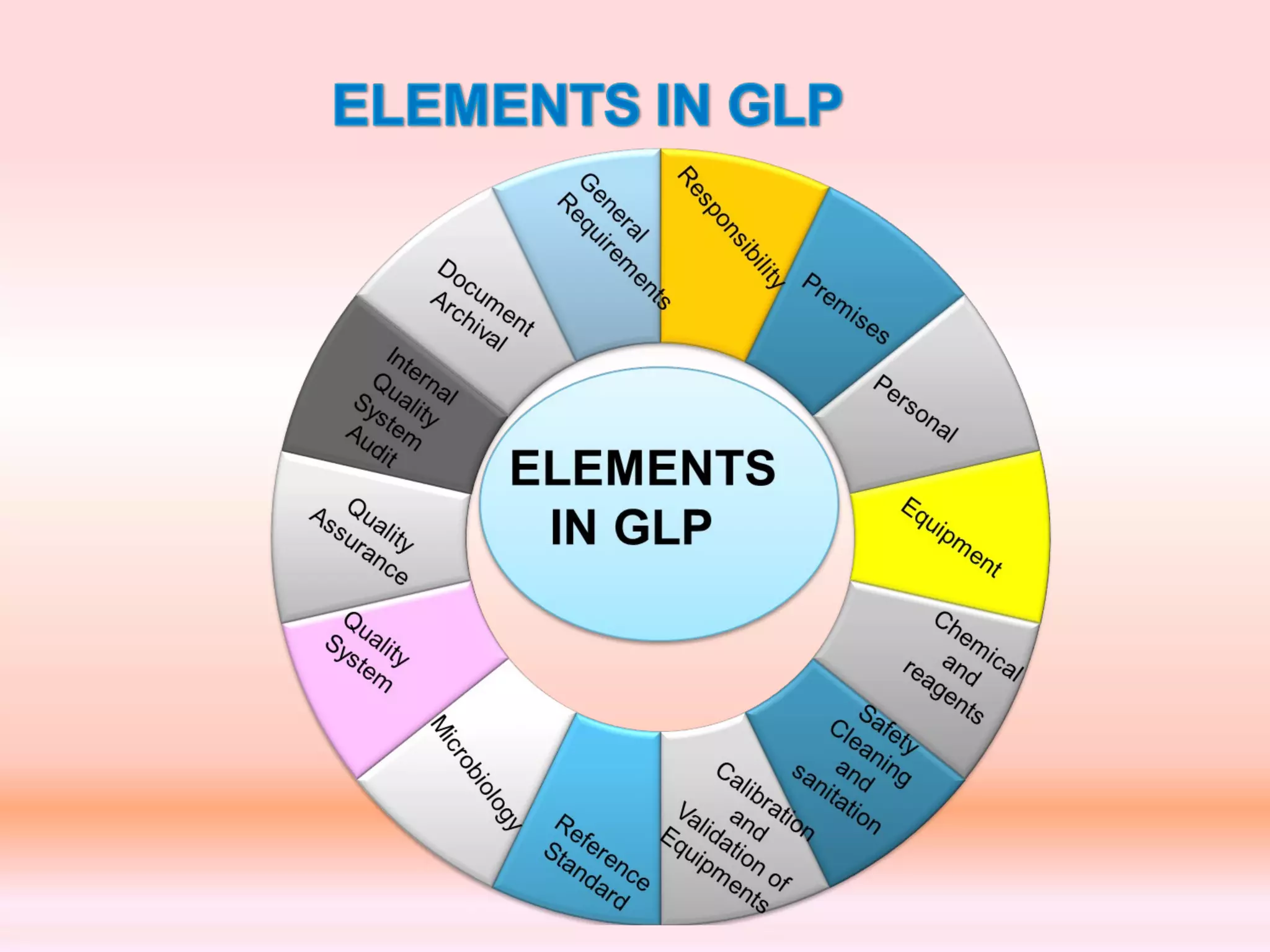
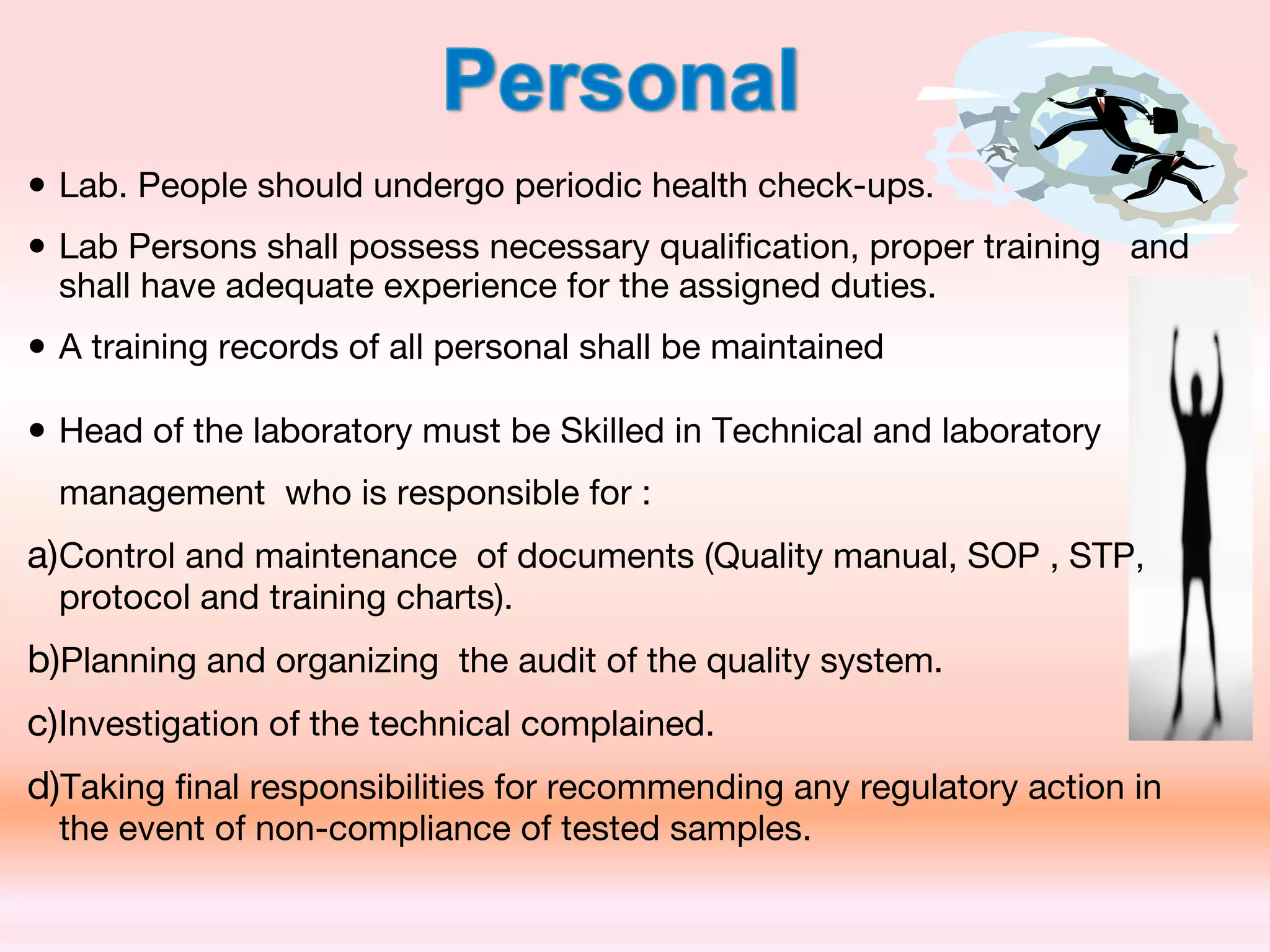
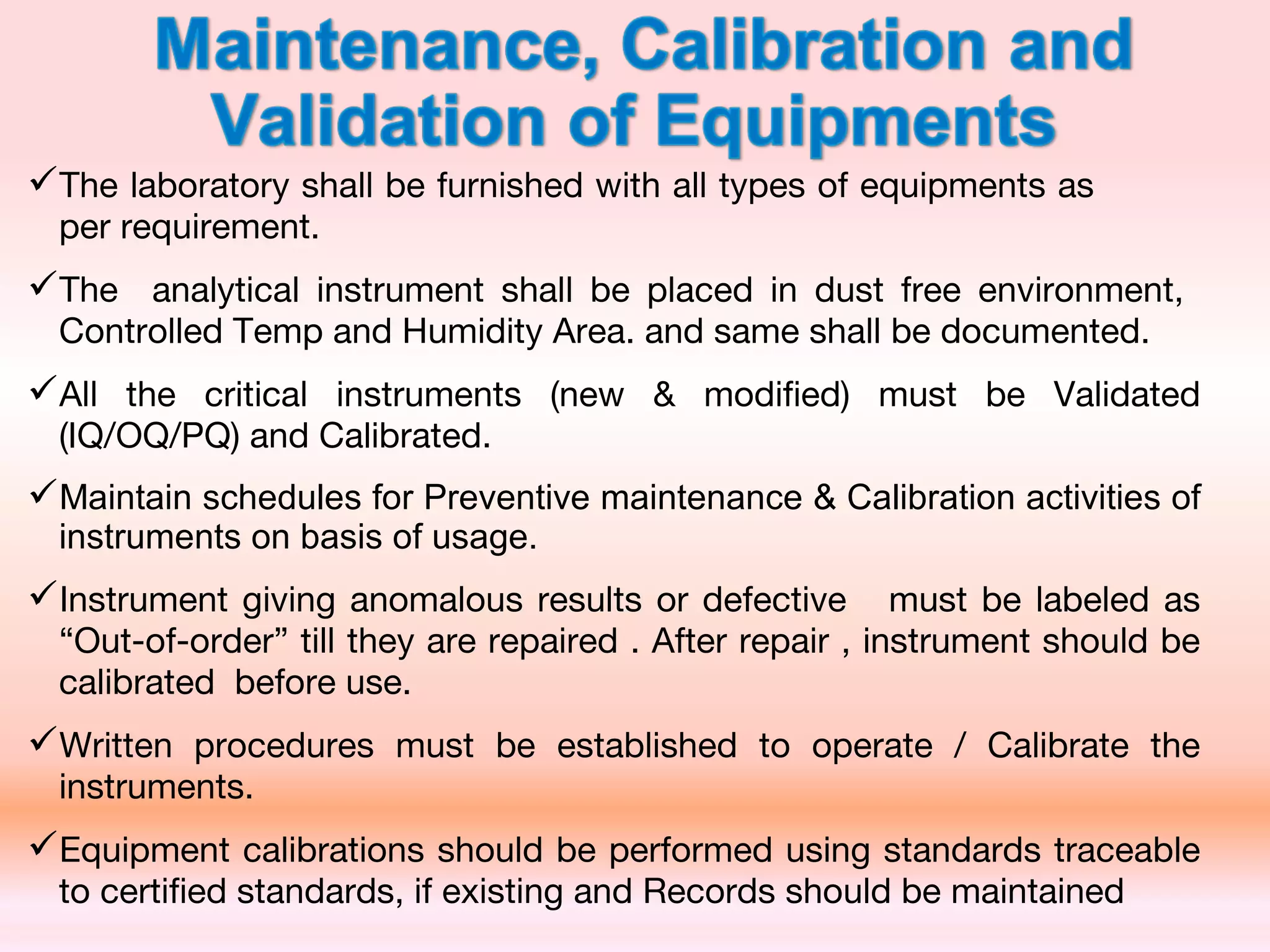
The document outlines Good Laboratory Practices (GLP) which were introduced in response to cases of fraudulent activities and poor lab practices discovered by the FDA in the 1970s. GLP helps ensure data submitted is a true reflection of study results and can be relied upon for risk assessments. It establishes standards for laboratory facilities, equipment, personnel qualifications, organization, standard operating procedures, test and control articles, protocol for conducting studies, and maintenance of raw data. Adhering to GLP helps assure regulatory authorities that submitted safety data is of high quality and integrity.