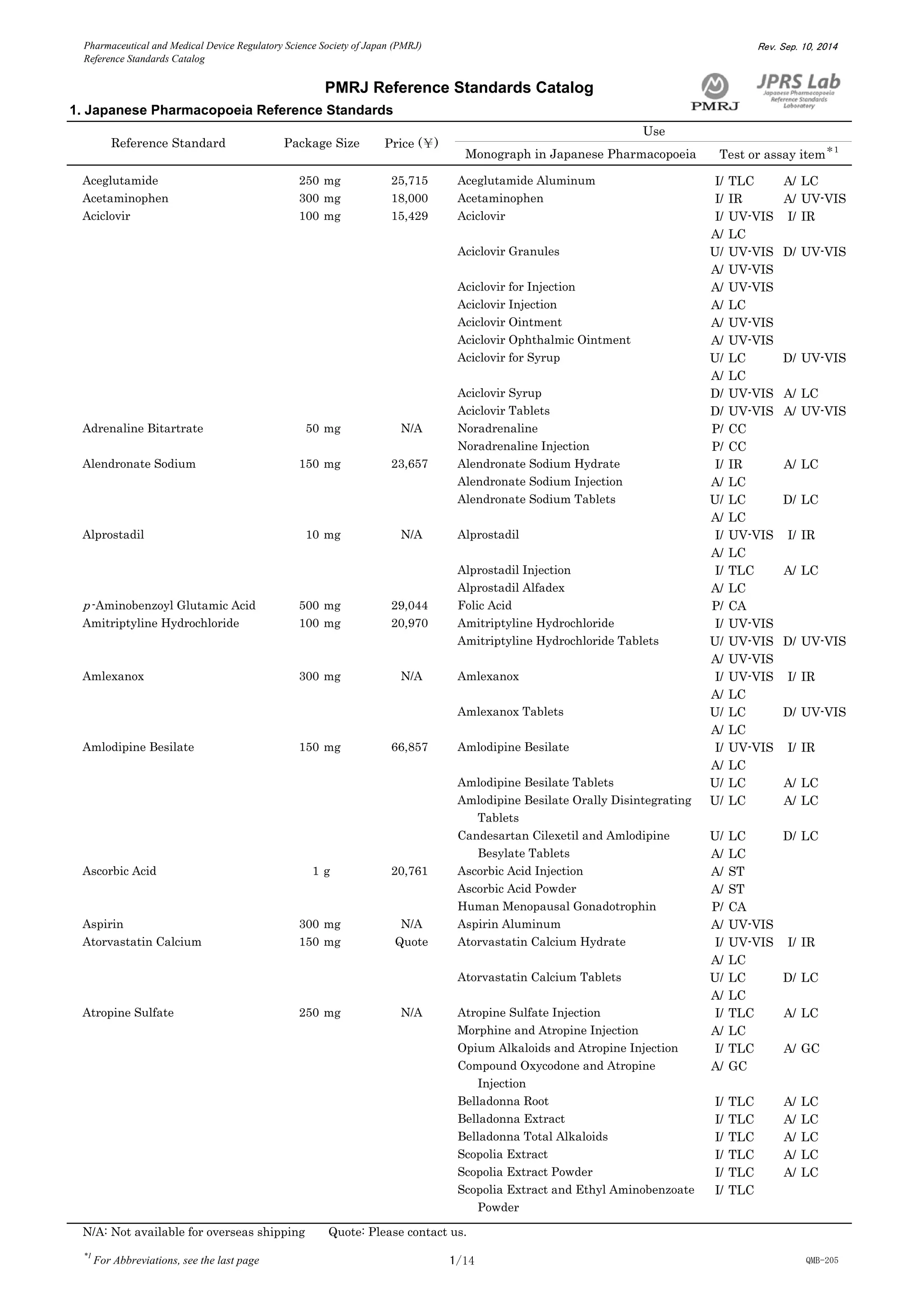
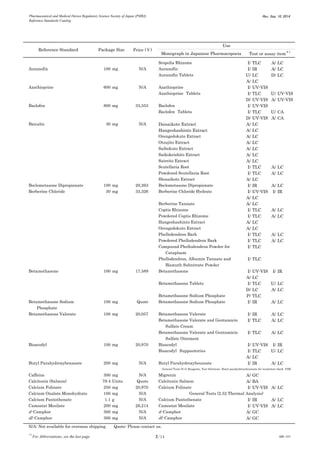
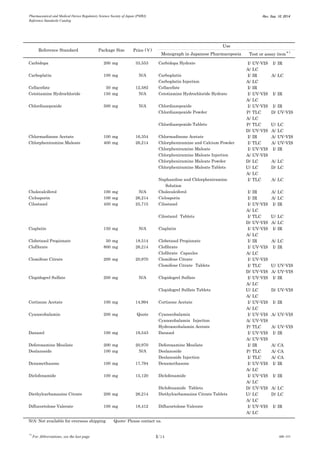
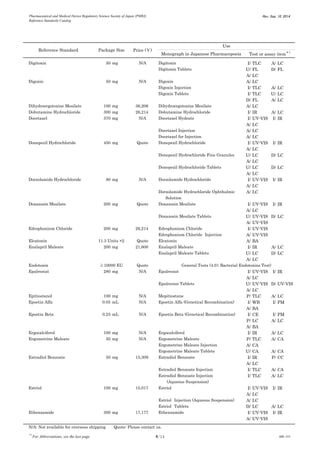
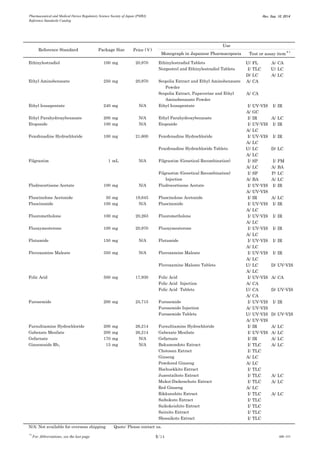
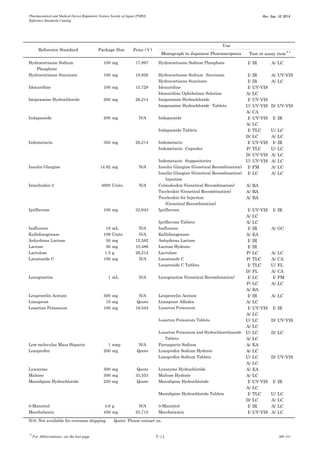
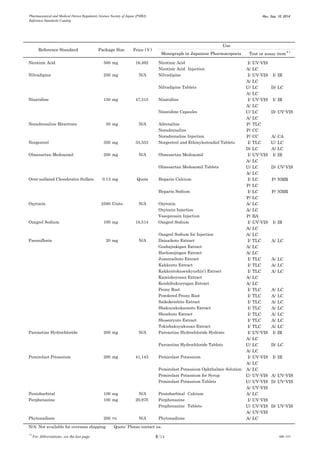
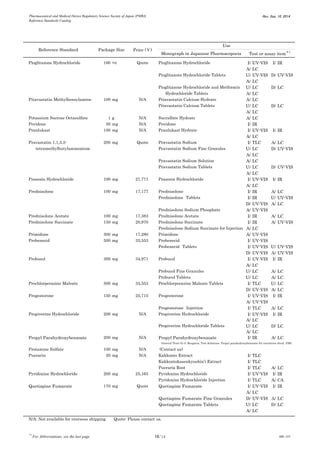
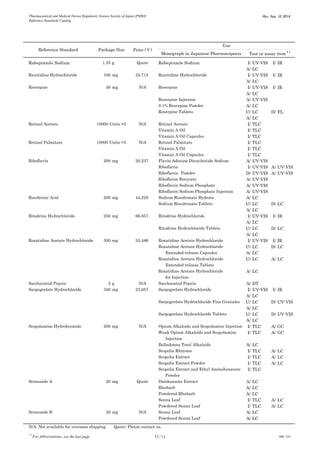
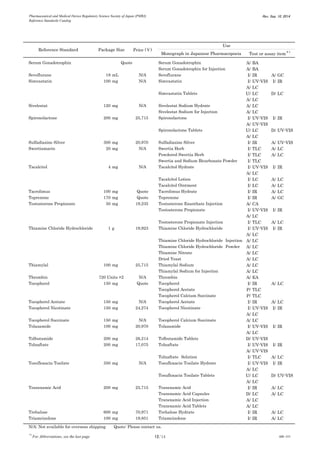
This document is a catalog from the Pharmaceutical and Medical Device Regulatory Science Society of Japan (PMRJ) listing reference standards. It provides information on over 200 reference standards including the reference standard name, package size, price in Japanese yen, intended use, and test or assay the standard can be used for according to the Japanese Pharmacopoeia monographs. The catalog is revised as of September 10, 2014.