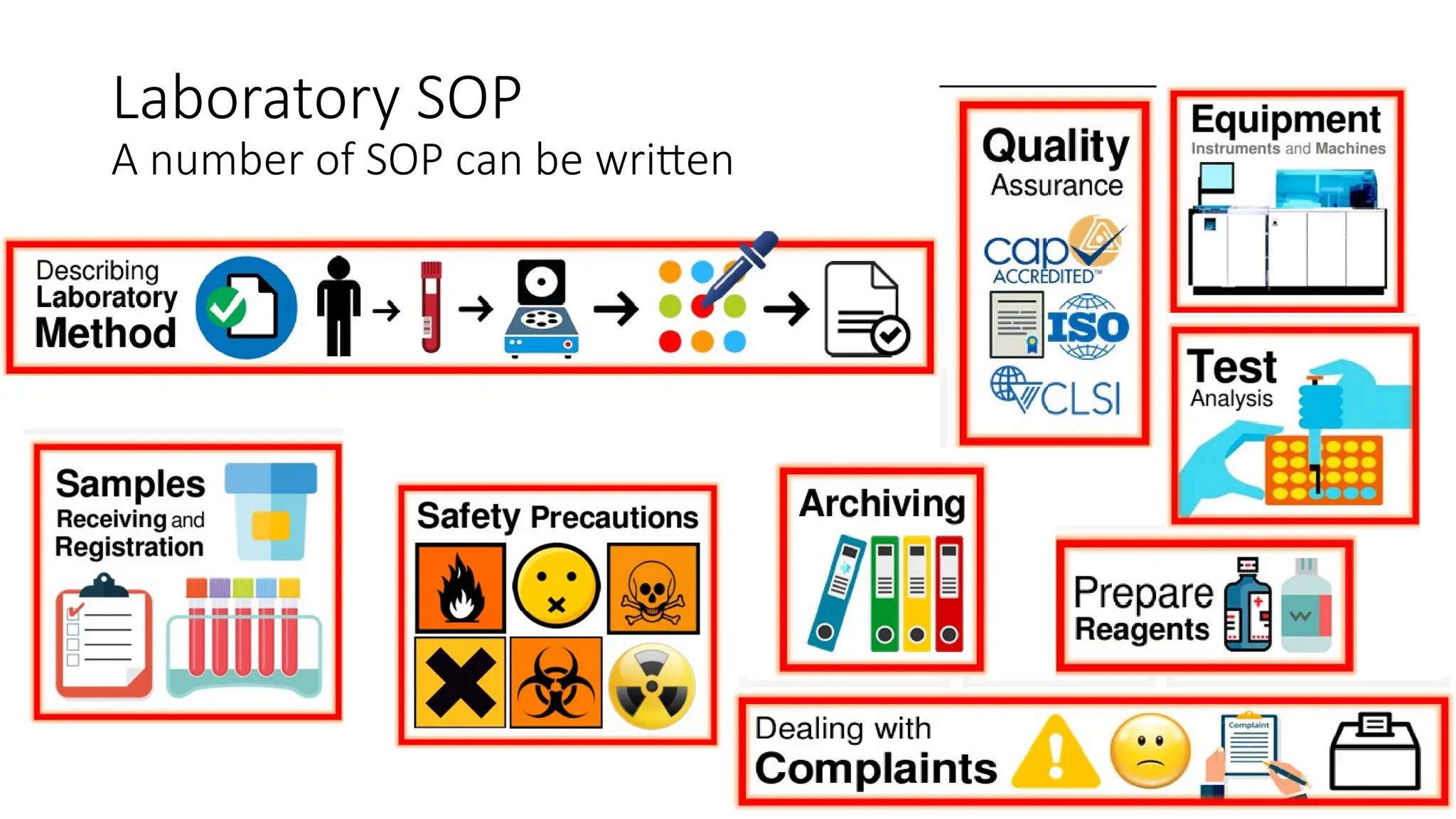
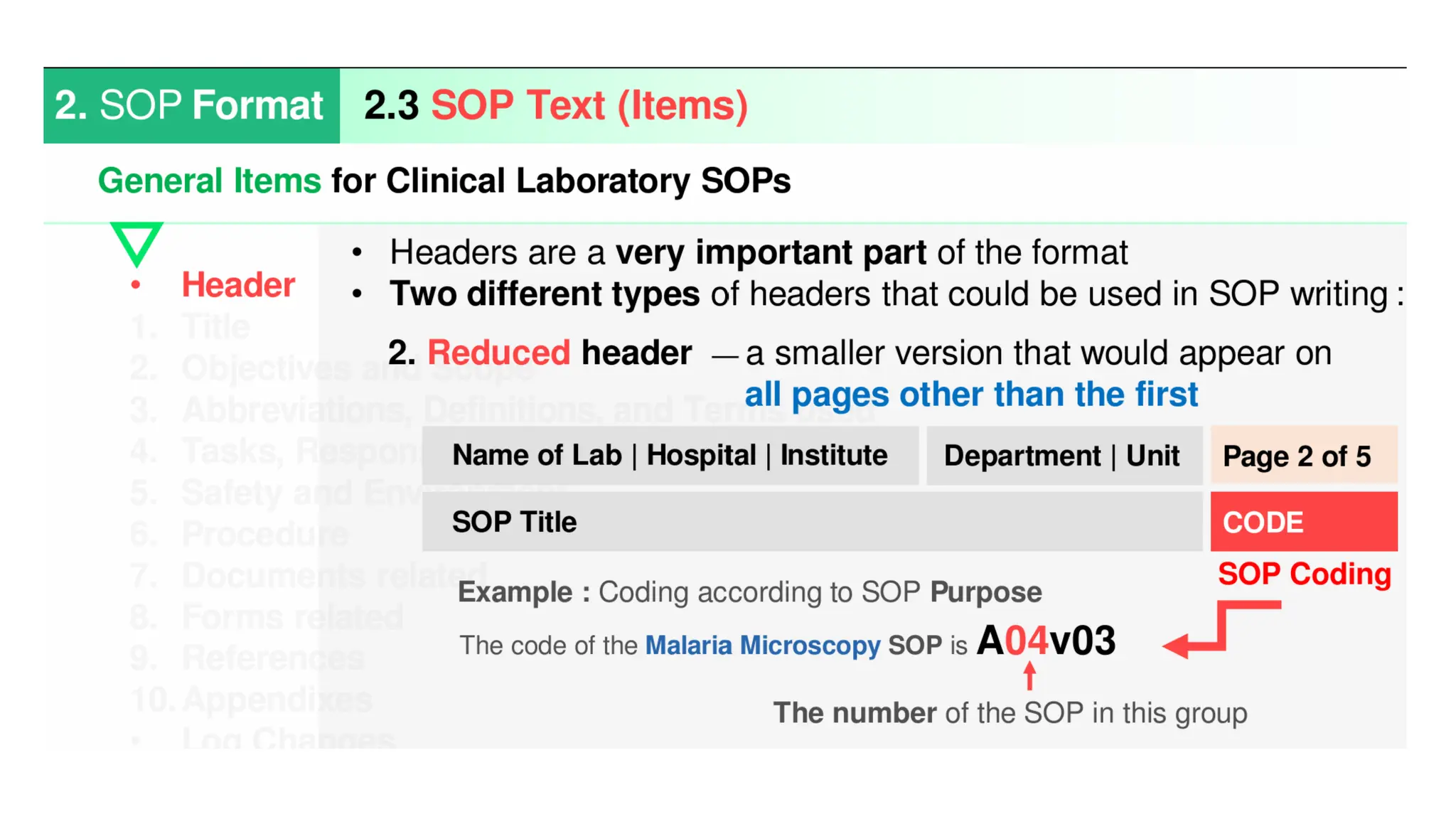
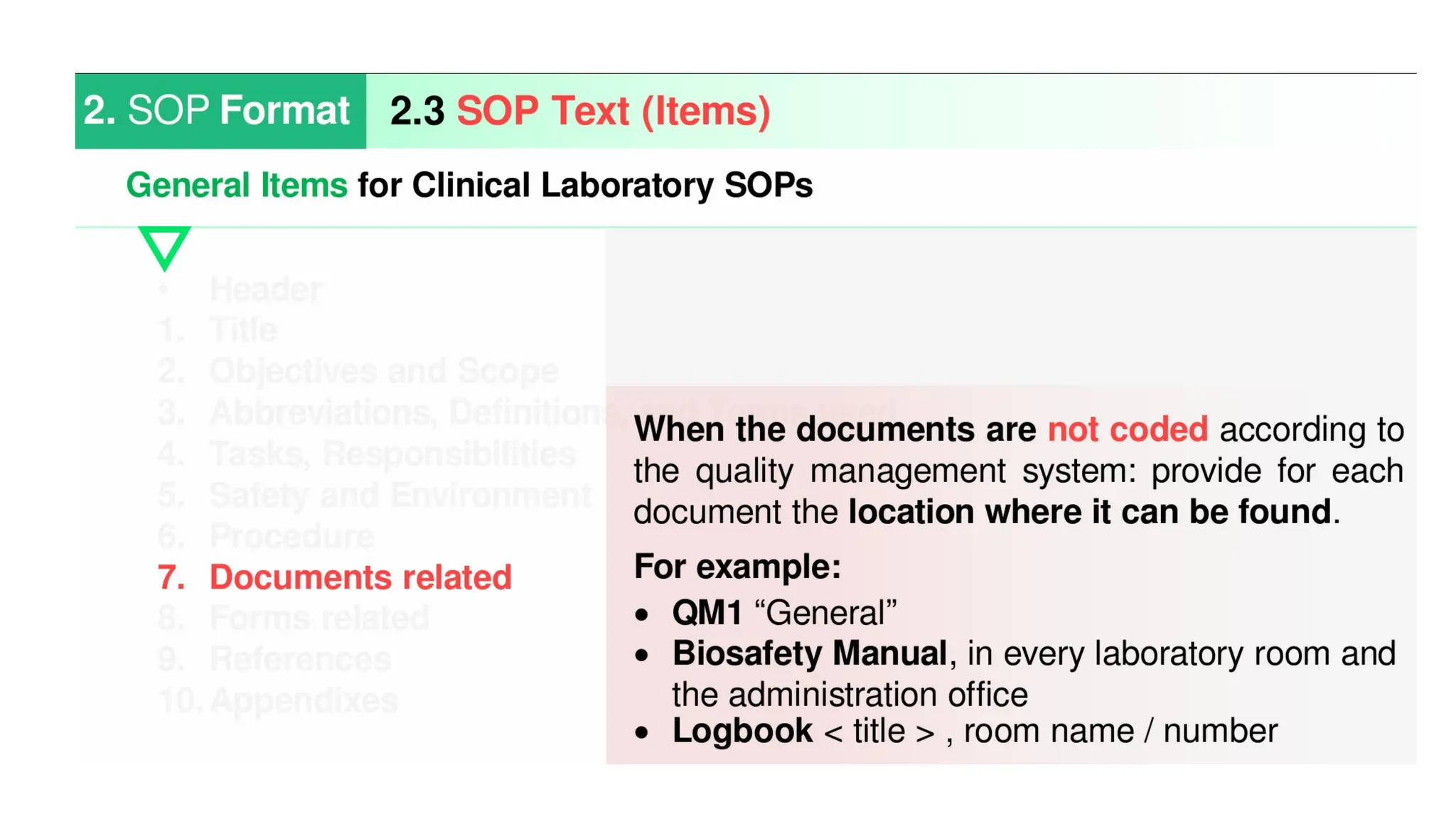
Standard Operating Procedures (SOPs) are essential written instructions that guide laboratory staff in performing routine activities consistently and accurately. They help ensure that tests are conducted the same way by all personnel, which leads to reliable results and facilitates monitoring patient changes over time. Effective SOPs are concise, clear, regularly updated, and include all necessary details for personnel to perform tasks correctly.