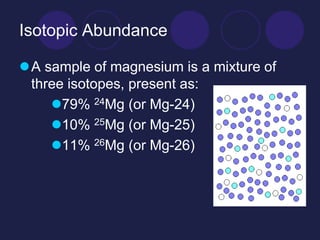
Magnesium has three naturally occurring isotopes: magnesium-24, magnesium-25, and magnesium-26. They have the same number of protons and electrons but different numbers of neutrons, giving them different atomic masses. Magnesium-24 makes up 79% of magnesium and has the greatest influence in calculating magnesium's average atomic mass of 24.3 amu.